October 9, 2018 report
Repurposing dimethyloxalylglycine to inhibit glutamine metabolism
A team of researchers from the U.K. and the U.S. has found that a drug used to study hypoxia can also be used to inhibit glutamine metabolism—a possible means for targeting cancer cells by cutting off their supply line. In their paper published in the journal Nature Chemical Biology, the group explains their study of the drug and its new possible use in targeting tumors. Barbara Nelson, Daniel Kremer and Costas Lyssiotis with the University of Michigan have written a News and Views piece on the work done by the team in the same journal issue.
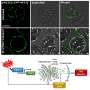
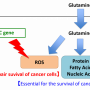
The researchers began their study by noting that a lot of tumors require glutamine to survive—they utilize it in a process called glutaminolysis. Prior research had revealed that glutamine can be involved directly in biosynthetic processes or it can be converted to glutamate. In the latter case, it becomes de-animated to α -Ketoglutarate (αKG)—the de-animation process is sometimes catalyzed by glutamate dehydrogenase (GDH), where KG and ammonia are produced, and at other times by transaminases. Also, noting that αKG is a key feature in relevant metabolic pathways, suggested that its analog, a drug called dimethyloxalylglycine (DMOG) might be useful in inhibiting glutamine used by cancer cells.
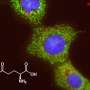
To find out if this might be the case, the researchers hydrolyzed DMOG, producing methyloxalylglycine (MOG), which incited cytotoxicity when transported by monocarboxylate transporter 2 (MCT2). They report that the end result was a decrease in tricarboxylic acid, which is derived from glutamine. They also found a reduction in mitochondrial respiration and a decrease in ATP production. A closer look showed that the use of MCT2 allowed MOG to gain entry to cells with high enough concentrations to slow the production of enzymes involved in glutamine metabolism. Thus, they report, MCT2 dictates how well NOG can perform in inhibiting glutamine metabolism.
The result of the work is the establishment of a new means of causing cytotoxicity, effectively reducing the utilization of glutamine by cells. The next step will be to find out if the inhibition of GDH and isocitrate dehydrogenases by NOG are alone responsible for the cytotoxicity observed or if there are other unknown elements at play.
More information: Louise Fets et al. MCT2 mediates concentration-dependent inhibition of glutamine metabolism by MOG, Nature Chemical Biology (2018). DOI: 10.1038/s41589-018-0136-y
Journal information: Nature Chemical Biology
© 2018 Phys.org