New study reveals proton hydration structures are asymmetric

How water solvates and transports protons is a fundamental question facing chemists and biologists alike and is vital to our understanding of processes such as photosynthesis and cellular respiration.
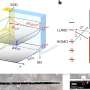
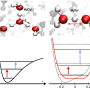
A team of researchers at the University of Chicago used broadband 2-D IR spectroscopy to reveal proton behavior when acids like HCl dissociate in water. Although general chemistry textbooks typically teach that the proton associates with water as a hydronium ion H3O+, they discovered that the proton is strongly bound between two water molecules and that the structures are predominantly asymmetrical.
"We are building off predictions made through computational modeling, including the work of Greg Voth, professor of chemistry at the University of Chicago, who is a leader in developing computational models for proton transfer in pure water and biological systems," said Joseph Fournier, assistant professor of chemistry at Washington University in St. Louis and former Arnold O. Beckman postdoctoral fellow at the University of Chicago. "However, the experimental and technical challenges faced in studying the proton in water has left these models unverified. We feel this study offers the most comprehensive experimental view into the complicated nature of how water interacts with protons."
The study was made possible through advances in the field of 2-D IR spectroscopy developed in the group of Andrei Tokmakoff, professor of chemistry at the University of Chicago and co-author on the paper. 2-D IR uses short infrared laser pulses to capture snapshots of the structures of molecules before they can move. Once able to capture a snapshot, researchers found that there were many structural variations possible when a proton was shared between two water molecules, and that these structures persist longer than previously thought.
"For example, the proton could be in the middle or a little skewed. Or the two water molecules could be varying distances from each other," said Fournier.
These data will now be used to improve computational models, which will help researchers quantitatively determine the nature of these structural distributions and understand why these structures persist longer than previously thought. Additionally, co-author and graduate student researcher William Carpenter intends to study how the structures evolve in time as the proton moves from one water molecule to the next.
Fournier also plans to apply the proton transport research in catalytic processes.
"A lot of chemists are trying to develop catalysts that mimic what plants do—splitting water for new clean energy sources," he said. "Catalytic processes like water splitting rely on multiple proton transfer events. Understanding how this works at the molecular level could help us use water as fuel."
More information: Joseph A. Fournier et al. Broadband 2D IR spectroscopy reveals dominant asymmetric H5O2+ proton hydration structures in acid solutions, Nature Chemistry (2018). DOI: 10.1038/s41557-018-0091-y
Journal information: Nature Chemistry
Provided by University of Chicago