New technologies for producing medical therapeutic proteins
Bacterial systems are some of the simplest and most effective platforms for the expression of recombinant proteins. They are more cost-effective compared to other methods, and are therefore of great interest not only for Lobachevsky University researchers, but also for manufacturers of therapeutically important drugs.
However, in addition to the target recombinant proteins, cells also produce a large number of endogenous proteins, including SlyD. It is a small protein consisting of three domains. Its C-terminal region is rich in histidine residues, and SlyD therefore exhibits a high affinity for the 2-valent ions and is purified together with the target proteins in the course of metal-affinity chromatography. This results in the need for additional purification steps, and as a consequence, increases the cost of the technological process for obtaining therapeutic recombinant proteins.
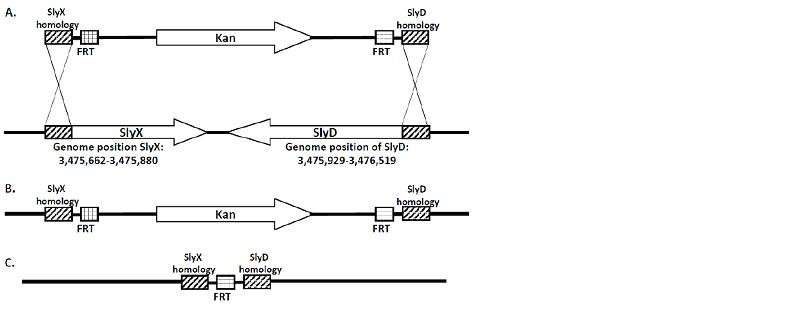
A team of Lobachevsky University researchers under Professor Viktor Novikov, Director of the UNN Center for Molecular Biology and Biomedicine, has obtained a series of E. coli strains deficient in the SlyD/SlyX genes. The strains were engineered using λ-red mediated chromosomal deletion. (Figure 1.)
"The sequence of SlyD/SlyX in the E. coli genome was replaced by a gene responsible for resistance to the antibiotic kanamycin that was flanked on both sides by FRT sites, from where it was later removed by FLP recombinase," Viktor Novikov notes.
Using the example of recombinant bispecific protein MYSTI-2 consisting of two modules that are active centers of antibodies against mouse proteins F4/80 and TNF, the scientists compared the activity of proteins isolated from the original and mutant strains. As a result of the study, it was determined that the removal from the E. coli genome of the SlyD and SlyX genes, which presumably encode chaperones that support the spatial structure of Escherichia coli proteins, does not result in a disruption of recombinant proteins' functional activity.
By obtaining original E. coli strains, the researchers were able to solve the problem of contamination of recombinant proteins and to ensure their successful single-stage purification by metal-affinity chromatography.
"The obtained set of slyD/slyX-deficient strains of E. coli can be used to produce in a pure form a wide range of prokaryotic and eukaryotic proteins, including medical therapeutic proteins. This makes the development and production of new medicinal and preventive biological preparations easier, simpler and cheaper," concludes Viktor Novikov.
More information: Vladislav V. Mokhonov et al, SlyD-deficient Escherichia coli strains: A highway to contaminant-free protein extraction, Biochemical and Biophysical Research Communications (2018). DOI: 10.1016/j.bbrc.2018.04.029
Journal information: Biochemical and Biophysical Research Communications
Provided by Lobachevsky University