KAIST to introduce enhanced PDT to cure cancer with fewer side effects
A KAIST research team developed near-infrared fluorophores-based photodynamic therapy (PDT) that reduced the downside of existing PDTs.
PDT is a way to cure wounds with lasers instead of drug treatment. When a laser irradiates a targeted site, a photosensitizer (PS) absorbs light energy and then converts oxygen to singlet oxygen or free radicals, leading to programmed cell death. This treatment has been used widely in clinical fields, especially for skin disease because it allows noninvasive treatment.
However, the existing PDTs have limitations for first-line therapy because PDT agents can cause genetic variations when they have low efficiency, hence reducing treatment effects.
The key to enhancing the efficiency of PDTs is how much PS can be concentrated to a wanted site, which laser wavelength the PS is reacted to, and how fast the PS clears organelle after treatment.
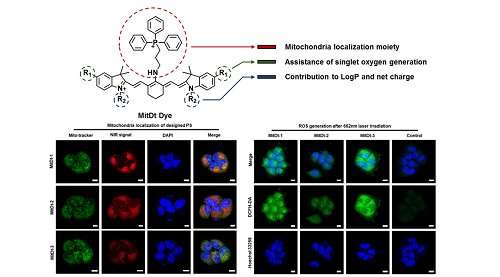
Professor Yeu-Chun Kim and his team from the Department of Chemical and Biomolecular Engineering, in collaboration with Professor Ji-Ho Park from the Department of Bio and Brain Engineering, developed a new PS called mitochondria targeting photodynamic therapeutic agent (MitDt) to maximize PDT effects while reducing unwanted side effects.
Mitochondria has emerged as target sites to maximize the effects of PS since they play essential roles in metabolism and have high transmembrane potential.
According to the team, when mitochondria is photodamaged by reactive oxygen species (ROS) generated after laser irradiation, they immediately lose their mitochondrial membrane potential and initiate apoptosis. Therefore, combining the PDT agent with the mitochondrial targeting agent can result in rapid damage to cancer cells, improving therapeutic efficacy and reducing unwanted side effects.
To successfully apply mitochondria-targeting PS, the team developed near-infrared (NIR) region PDT agents, which can be used to treat deep-tissue level cancer due to the permeability of the NIR laser. Light scattering is also decreased, thus obtaining higher therapeutic efficacy.
However, there is a problem of generating singlet oxygen when irradiating with an NIR laser. To address this issue, the team developed a novel PS that combines a functionalized NIR dye and a mitochondria-targeting agent to gain the benefit of rapid organelle clearance after treatment and also remain in cancer mitochondria for a long time, amplifying the amount of ROS to the target sites irradiated by the laser.
To verify the efficacy, the team injected MitDt into tumor-bearing mice. They were irradiated with an NIR laser at 662 nm to induce cancer treatment and their cancer size was reduced up to three-fold.
Ph.D. candidate Ilkoo Noh, who led this research said, "This enhanced photodynamic cancer treatment has the advantage of treating a wanted site without any side effects because this PS stays longer in a mitochondrial cancer cell. We also confirmed that the PS did not cause cytotoxicity."
Professor Kim added, "This research outcome will reduce the danger of side effects and can be applied for treating various diseases."
More information: Ilkoo Noh et al. Enhanced Photodynamic Cancer Treatment by Mitochondria-Targeting and Brominated Near-Infrared Fluorophores, Advanced Science (2017). DOI: 10.1002/advs.201700481