New electrocatalyst developed for oxygen reduction reaction
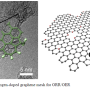
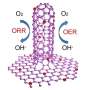
Recent research published in a paper in NANO by a team of researchers from Beihang University have fabricated a new type of VNQD-NG as nonprecious metal-based electrocatalyst for oxygen reduction reaction (ORR). The unique structural features of plentiful VN quantum dots with the sizes of 3-6 nm, high surface area and multi-level pores afford considerable structural edges and defects as active sites, maximizing the exposed active sites and providing sufficient electron transport pathways for ORR. This has significant practical and commercial applications.
A team of researchers from Beihang University in Beijing, China has demonstrated a new type of low-cost nonprecious metal-based catalysts as an alternative to platinum-based catalysts for oxygen reduction reaction. In their work, a new type of vanadium nitride quantum dots anchored homogeneously onto nitrogen-doped graphene (denoted as VNQD-NG) is fabricated by a simple hydrothermal method and subsequent ammonia annealing process. The VNQD-NG exhibits high electrocatalytic activity, long durability and high selectivity for ORR, superior to commercially available Pt-C. Their report appears in the forthcoming issue of the journal NANO.
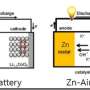
Developing suitable electrocatalysts for ORR is significant to the practical applications of fuel cells and metal-air batteries due to the kinetic sluggishness of ORR with a complex four-electron transfer process. Despite the high efficiency of platinum (Pt) and Pt-based alloys for ORR, their high cost along with the rare reserves in nature largely impedes the large-scale practical application. Therefore, developing low-cost nonprecious metal-based catalysts as an alternative to Pt-based catalysts are crucial. Vanadium nitrite (VN) not only has the similar structure and high density of states to those of above transition-metal nitrides, but also shows good corrosion resistance and high electrical conductivity, holding great promise as an active electrocatalyst for ORR.
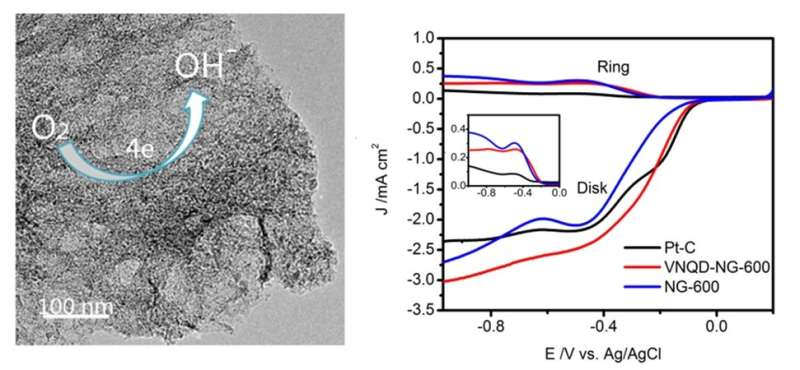
The as-prepared VNQD-NG possesses numerous zero-dimensional (0D) VN quantum dots (QDs) with the sizes of 3?6 nm anchored homogeneously onto the surface of nitrogen-doped graphene, affording considerable structural edges and defects as active sites for ORR. Moreover, the VNQD-NG nanosheets can simultaneously construct a three-dimensional (3-D) porous architecture to maximize the exposed active sites and provide sufficient electron transport pathways during ORR. Researchers executed cyclic voltammogram (CV) measurements in an aqueous 0.1 M KOH solution to investigate the ORR characteristics of VNQD-NG samples, reflecting a prominent electrocatalytic activity of the VNQD-NG for oxygen reduction. Chronoamperometric measurements show that a strong current response occurs at Pt-C catalyst when 3 M methanol was injected in oxygen-saturated solution, whereas the VNQD-NG maintains a stable current without any distinct response. Apparently, the VNQD-NG hybrid exhibits a high selectivity to methanol in the alkaline electrolyte, tolerance to crossover effects caused by fuel molecules permeating through the polymer membrane. Researchers also conducted the rotating ring-disk electrode (RRDE) measurements to verify the ORR electrochemical kinetics of VNQD-NG, displaying a comparable ORR onset potential to that of Pt-C catalyst, and higher electron transfer number (n) and kinetic-limiting current density (Jk) than that of commercial Pt-C and other non-Pt catalysts. The current-time (i?t) choronoamperometric response for VNQD-NG reveals a quite low attenuation of 77 % after 30 000 s, which provides further evidence that the stability of VNQD-NG is superior to that of commercial Pt-C (60 %).
VNQD-NG exhibits high electrocatalytic activity, high selectivity and long durability for ORR, better than commercially available Pt-C. These achievements could provide an extension of developing various other 3-D porous metal nitride quantum dots onto graphene for broad applications in sensors, catalysis, and other electronic devices.
More information: Jing Wang et al, VN Quantum Dots Anchored Uniformly onto Nitrogen-Doped Graphene as Efficient Electrocatalysts for Oxygen Reduction Reaction, Nano (2018). DOI: 10.1142/S1793292018500418
Provided by World Scientific Publishing