Possible breakthrough in understanding how antibiotics treat bacteria
Scientists from Newcastle University and the UK's ISIS Neutron and Muon research facility have worked together on a new project that is increasing our understanding of how antibiotics treat bacteria.
With the number of antibiotic resistant bacteria increasing in recent years, the ability to develop a way to combat this resistance could be essential for our future health.
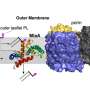
The team used a technique known as neutron reflectometry at STFC's ISIS facility in Oxfordshire to examine how Polymyxin B, a last resort antibiotic, interacts with the outer membrane of Gram-negative bacteria. These hardy bacteria are responsible for life-threatening diseases like pneumonia and meningitis, making them key targets for clinical research.
The more scientists can learn about these bacterial membranes, and the interactions of drugs with them, the greater chance we have of targeting the threat of antibiotic resistance and the impact on our ability to treat disease.
Understanding the interactions of such antibiotics with the outer membrane of Gram-negative bacteria is of vital importance because four out of six organisms responsible for the majority of hospital acquired infections, are Gram-negatives.
Professor Jeremy Lakey, from the Institute for Cell and Molecular Biosciences at Newcastle University, said: "Most antibiotics currently used to kill Gram-negatives enter the bacteria via protein pores which can mutate to resist the drug. Polymyxin B kills bacteria by directly disrupting the outer membrane structure. Exploiting such different types of drug molecules is one strategy we can use to overcome the antibiotic resistance that has built up over the years."
"Targeting the outer membrane offers alternative ways to kill pathogens and our new data shows us why bacteria are vulnerable to this type of attack. They need to keep their outer membrane flexible to grow but that provides the weakness exploited by the Polymyxin."
In a world-first, the team were able to use an artificial model of the outer membrane to not only explain the temperature dependence of Polymyxin B function but also support the notion that bacteria actively control the viscosity of their outer membrane as growth temperatures vary.
The model – created in 2013 using neutron technology at the ISIS Neutron and Muon Source – provides a realistic framework that accurately reproduces the essential asymmetric structure and impermeability of the Gram-negative outer membrane.
Using this model, the team were able to mimic both the initial insertion and subsequent process by which the antibiotic disrupts the structure of the outer membrane and increases membrane permeability, leading to cell death.
Dr. Luke Clifton, ISIS Neutron & Muon Source said: "The outer membrane of the Gram negative bacteria is the barrier between these microorganisms and their external environment. The models of this surface, which we have developed with our colleagues at Newcastle University, allow us to gain molecular level details on this barrier in conditions found in the living organism.
"Neutron science is key to these studies as neutrons are a highly adaptable, non-damaging probe of molecular structure which can differentiate between the different biomolecules found in these complex systems. This allows us to locate the individual components within the bio-membrane and examine how they change during the interaction of the antibiotic."
The INTER instrument at ISIS used by the team allowed for higher resolution and enhanced measurement speeds meaning the scientists could accurately investigate the dynamics of biological systems.
The team now plan to investigate how bacteria develop resistance mechanisms against Polymyxin which will help in the design of second generation molecules, to use when Polymyxin itself becomes less effective.
More information: Nicolò Paracini et al. Liquid crystalline bacterial outer membranes are critical for antibiotic susceptibility, Proceedings of the National Academy of Sciences (2018). DOI: 10.1073/pnas.1803975115
Journal information: Proceedings of the National Academy of Sciences
Provided by Newcastle University