Enzyme boost could hasten production of biofuels and other bioprocessed materials
Imperial scientists have enhanced the process of using biology to make products such as fuels, plastics, medicines, and cosmetics.
This could lead to cheaper and more environmentally friendly biofuel production and more efficient plastic recycling.
Bioprocessing, which uses living cells or their components to make products such as biofuels, plastics, medicines, and cosmetics, is time consuming and expensive.
Now, Imperial scientists say they can break down plant-based biomass 30 times faster than currently possible.
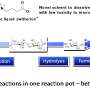
Dr. Alex Brogan, of Imperial College London's Department of Chemical Engineering, and colleagues modified the glucosidase enzyme, which helps break down complex carbohydrates in biomass, like cellulose from plant cells, into its basic units, glucose. The glucose can then be fermented to make ethanol, a form of biofuel.
Releasing glucose from cellulose is currently the most expensive and time consuming part of the process. This is partly because enzymes typically stop working at temperatures higher than 70 °C and when in industrial solvents like ionic liquids. However, if the enzyme could work in higher temperatures and ionic liquids, the conditions would hasten the process.
To make glucosidase more robust, Dr. Brogan and colleagues altered its chemical structure to let it withstand heat of up to 137 °C. The alteration also meant they could use the enzyme in ionic liquids instead of the usual water, and that they could use one enzyme instead of three.
They found that the combined effect of heat resistance and solubility in ionic liquids increased the glucose output 30-fold. If the technique is taken up on a large scale, fuel-related carbon emissions could fall by 80-100 per cent.
The findings are published today in Nature Chemistry.
Lead author Dr. Brogan said: "We've made bioprocessing faster, which will require less equipment and will reduce carbon footprint. One major advantage of this will be increased biofuel production—potentially helping biofuels become more widespread as a result."
Biofuels are fuels made from living matter like plants, otherwise called biomass. They are better for the environment than fossil fuels such as coal and gas because they are made from renewable sources and emit far less overall carbon dioxide.
Senior author Dr. Jason Hallett, also from Imperial's Department of Chemical Engineering, said: "Using biofuels made from corn starch, trees and other plant matter for vehicles and even electricity generation could massively reduce carbon emissions."
The alteration could be applied to a wide variety of enzymes, for a wide range of applications, such as making fuels from waste and recycling plastics, and can make bioprocessing more efficient.
"Non-aqueous homogenous biocatalytic conversion of polysaccharides in ionic liquids using chemically modified glucosidase" by Alex P. S. Brogan, Liem Bui-Le and Jason P. Hallett, is published on 15 June 2018 in Nature Chemistry.
More information: Non-aqueous homogenous biocatalytic conversion of polysaccharides in ionic liquids using chemically modified glucosidase, Nature Chemistry (2018). DOI: 10.1038/s41557-018-0088-6 , www.nature.com/articles/s41557-018-0088-6
Journal information: Nature Chemistry
Provided by Imperial College London