Gold protein clusters could be used as environmental and health detectors

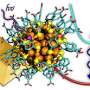
Peng Zhang and his collaborators study remarkable, tiny self-assembling clusters of gold and protein that glow a bold red. And they're useful: protein-gold nanoclusters could be used to detect harmful metals in water or to identify cancer cells in the body.
"These structures are very exciting but are very, very hard to study. We tried many different tools, but none worked," says Zhang, a Dalhousie University professor.
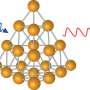
But synchrotron X-ray Absorption Spectroscopy, or XAS, done at the Canadian Light Source and its partner facility CLS@APS, provided the necessary insight to identify the surprisingly elegant structure of the glowing protein gold nanoclusters.
"Synchrotron XAS is the perfect tool, because it is very flexible, and can give you structural information about specific elements," he says.
The structure of these clusters had been a long-standing question in the field, in part because of their potential uses.
In the lab, researchers have shown that introducing other metals to the protein gold solution can quench the nanogold's bold glow, a property that could be used to detect metal pollution in rivers and other water bodies. Where metals existed in a water sample, the nanogold would stop glowing.
Even more exciting are the potential health applications for these clusters.
Nanogold and proteins are a natural fit for health technology, since proteins are a naturally-occurring part of the human body, and gold is completely non-toxic (which is why it can be used to decorate chocolates and can be found in some types of schnapps)
Coupled with the right "detector" or "linker" proteins, glowing nanogold could be used to precisely visualize, for instance, cancerous tumours. The right linker protein would simply attach nanogolds to diseased cells, which could then be located with a fluorescence microscope.
All of this is possible because of the nanogold's ability to glow.
Gold as we usually know it, in bulk, doesn't glow, or luminesce, under UV light. Add protein as a base for gold nanoclusters, and they will glow a bright red under UV light. In fact, the nanogold is a million times more luminescent than bulk gold.
This gold luminescence is enabled by its specific structure in protein gold nanoclusters: what Zhang describes as a "beautiful and surprising structure" of ten gold atoms, forming two interlocked rings.
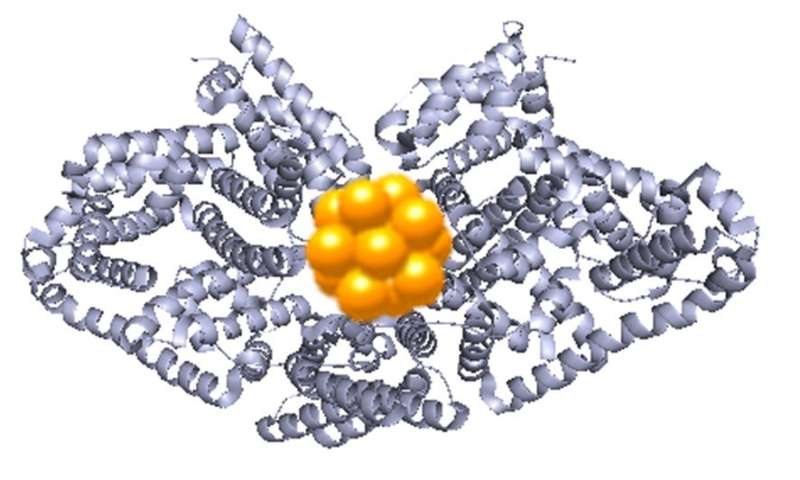
The protein acts as a scaffold of sorts, giving the gold a strong structure with strengthens its glow.
These clusters can self-assemble under the right conditions, offering a low-cost, low-energy production method.
A typical nanocluster is made with a precise array of chemicals put through specific steps, and might result in pollution. The production for these clusters, by contrast, couldn't be simpler.
Zhang's team heated a mixture of a commercial gold compound and proteins in water up to body temperature, 37C. And after 10-20 hours, the luminescent nanogold clusters formed. No other steps are required to create the luminescent clusters.
The process is known as green chemical synthesis, and cuts out the pollution that could otherwise be associated with these clusters.
"We used the synchrotron to follow how these beautiful structures are formed within the protein," says Zhang. The protein essentially works as a mini reactor, with its cysteine amino acids linking to the gold molecules and pulling them into shape. These experiments were conducted by Daniel Chevrier, a Ph.D. student in the Zhang's group.
The team also created clusters more conventionally, to verify their technique.
"If you don't use protein, yes you get a very similar structure, but you don't see the strong fluorescence. What happened?" asks Zhang.
To answer this question, his team compared conventionally-produced and self-assembled gold clusters, both with and without proteins.
Using the synchrotron, they showed that the protein not only enables self-assembly, but holds the clusters in place.
"Essentially the protein freezes the gold cluster so they cannot freely move, and then you can see the strong fluorescence," says Zhang. A freely-moving cluster, by contrast, twists and moves, which weakens the fluorescence.
"We are so excited about these new findings. Both gold and protein are highly interesting materials and when you combine these two you get something even more interesting, and potentially useful," says Zhang.
More information: D. M. Chevrier et al. Structure and formation of highly luminescent protein-stabilized gold clusters, Chemical Science (2018). DOI: 10.1039/C7SC05086K
Journal information: Chemical Science
Provided by Canadian Light Source