Evolving a more versatile CRISPR-Cas9
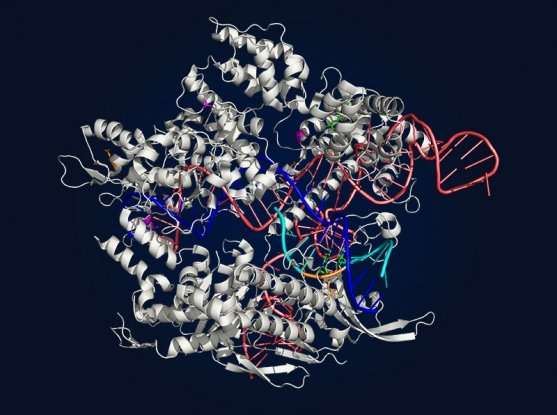
For all of Cas9's potential in research and therapeutics, it—as well as other enzymes in the CRISPR-associated family—has limitations. In order to recognize and bind to a DNA sequence, Cas9 needs a particular stretch of base letters to accompany the target. This requirement makes much of the genome inaccessible to the enzyme, significantly reducing its range of applications.
But a team of researchers at the Broad Institute of MIT and Harvard, Harvard University, and the Howard Hughes Medical Institute (HHMI) has evolved new variants of Cas9 that are compatible with a wider range of required bases, opening access to more regions of the genome. What's more, these new versions of the protein edit their targets far more accurately than prior versions.
The findings are described this week in Nature.
"We partially lifted the sequence limitation by broadening the compatibility of Cas9 so it can 'see' more of the genome," says senior author David Liu, the Richard Merkin Professor, director of the Merkin Institute of Transformative Technologies in Healthcare, and core institute member at the Broad Institute, professor of chemistry and chemical biology at Harvard University, and HHMI investigator. "There are numerous efforts to develop CRISPR technologies for clinical and research applications, and we hope that this work increases the likelihood that a particular site of interest is accessible."
The sequence of bases that Cas9 traditionally requires is called a PAM, for "protospacer-adjacent motif." Ultimately, if a PAM doesn't exist near the site of interest in the genome, natural CRISPR enzymes can't access it. Even the most versatile Cas9 enzyme found to date, SpCas9 from the bacteria Streptococcus pyogenes, can't access almost 95 percent of the genome for any of its applications (including turning genes on or off, DNA cutting, or base editing). That includes about three-quarters of the single-base mutations known to be associated with human disease.
"The PAM requirement, even for SpCas9, imposes a serious bottleneck on CRISPR use," says Liu. "If you're trying a CRISPR application that requires very precise positioning of the protein, you need to be lucky to have a PAM in just the right place."
To create SpCas9 variants with more PAM options, the team used a system called phage-assisted continuous evolution (PACE), developed in Liu's lab at Harvard in 2011. In an ecosystem of engineered bacteriophages and bacteria, PACE applies evolutionary pressure to encourage the phages to generate proteins with desired traits.
In this case, the phages carried the Cas9 gene into the bacteria, and the bacteria in turn copy and mutate the Cas9 DNA. Previous research has tinkered with specific sections of Cas9 (usually parts of the protein that are known to contact DNA or the guide RNA), but PACE is unique and unbiased in that it allows mutations to occur across the entire gene.
The system was engineered so that the phages could only replicate if the Cas9 gene picked up mutations granting the ability to bind additional PAMs. Once it did, the phages could pass that version of the gene to a new generation—which would infect more bacteria and repeat the cycle. PACE works more than 100 times faster than other protein evolution methods, with minimal human effort during the evolution process.
PACE turned out many forms of laboratory-evolved Cas9, or "xCas9," but the most optimal one opened up access to sites in roughly a quarter of the genome, including almost 75 percent of the point mutations associated with human disease. Liu and his colleagues showcased xCas9 in a variety of applications, including turning genes on, cutting DNA, and editing single base pairs (both C•G to T•A conversion and A•T to G•C conversion).
And most remarkably, even though xCas9 could see more sites in the genome, it made many fewer mistakes than nature's SpCas9 across the board.
"Scientifically, the increased specificity was the biggest shock," says Liu. "We speculate, though, that wild-type Cas9's off-target activity is a protective adaptation for bacteria to fight off different variations of viruses—and nature would have had to work pretty hard to get the exact right combination of amino acids so Cas9 could tolerate a few mismatches. So the enzyme might be poised to lose that promiscuity if you mutate it enough."
The researchers are collaborating with other labs to determine the molecular basis of xCas9's higher DNA specificity, and continuing to use the PACE platform to evolve new enzyme variants with additional PAM compatibilities.
"The hope is that, eventually, we'll have a big bookshelf of Cas9 or other CRISPR enzyme solutions," says Liu. "Our efforts in this line of research are never really over until we can access every possible site in the human genome."
More information: Johnny H. Hu et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity, Nature (2018). DOI: 10.1038/nature26155
Kevin M. Esvelt et al. A system for the continuous directed evolution of biomolecules, Nature (2011). DOI: 10.1038/nature09929
Journal information: Nature
Provided by Broad Institute of MIT and Harvard