Researchers create 3-D structure of the nuclear pore complex
For the first time, researchers have produced a nearly complete three-dimensional structure for the yeast Nuclear Pore Complex (NPC). This discovery represents a major step toward identifying the atomic structure of the NPC, which soon may provide researchers with a better understanding of how the central transport channel functions.
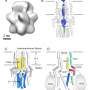
The NPC is the largest channel in the cell and spans the double membrane of the nuclear envelope. This remarkable channel provides a gateway to transport macromolecules back and forth between the nucleus and the cytoplasm. Due to its large size and dynamic nature a full structural and functional understanding of the NPC had been impeded until now.
The structure of the NPC was determined using a novel Integrative Modeling approach in which information from a large number of different experiments was used to computationally determine a set of models that best fit all the input data. The sheer size and complexity of the channel required data contributed directly from nine laboratories. In total, researchers were able to accurately place 552 NPC proteins, known as nucleoporins, within this large channel which is shaped somewhat like a wagon wheel with eight major spokes that connect the core scaffold to a more flexible central channel region (the central transporter).
"This transport gateway provides a control point to regulate development and cell growth. Unraveling the architecture of this mammoth transport machine provides us with a great deal of insight into how this channel is constructed and suggests how it may function," explained corresponding author Christopher Akey, PhD, professor physiology and biophysics at Boston University School of Medicine.
According to the researchers, these findings may one day help explain changes in cancer cells.
The findings appear in the journal Nature.
More information: Integrative structure and functional anatomy of a nuclear pore complex, Nature (2018). nature.com/articles/doi:10.1038/nature26003
Journal information: Nature
Provided by Boston University School of Medicine