Full-length serotonin receptor structure seen for first time
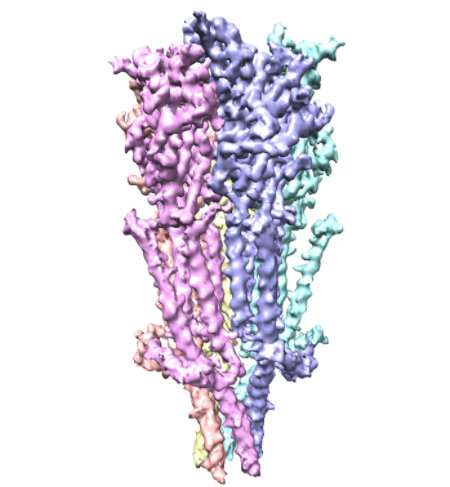
A team of researchers from Case Western Reserve University School of Medicine have used Nobel prize-winning microscope technology to see full length serotonin receptors for the first time. The tiny proteins—approximately a billionth of a meter long—are common drug targets, despite limited available information about their structure. Now, new images published in Nature Communications provide snapshots of the receptors, including details about molecular binding sites that could lead to more precise drug design.
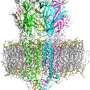
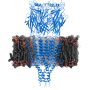
Serotonin receptors sit in cell membranes throughout the body, including the brain, stomach, and nerves. They are highly dynamic with many moving parts, making them difficult subjects to capture. Researchers commonly break the receptor into pieces to study it. But by studying full-length serotonin receptors, researchers in the new study showed how its different portions interact. The researchers describe "a finely tuned orchestration of three domain movements" that allows the receptors to elegantly control passageways across cell membranes.
The study reveals how serotonin receptors work, says study first author Sandip Basak, PhD, postdoctoral fellow in the department of physiology and biophysics at Case Western Reserve University School of Medicine. "The serotonin receptor acts as a gateway, or channel, from outside the cell to inside," he says. "When serotonin binds onto the receptor, the channel switches conformation from closed to open. It eventually twists into a 'desensitized' state, where the channel closes but serotonin remains attached. This prevents it from being reactivated." Different conformations help serotonin receptors serve as "gatekeepers" in cell membranes.
Drugs that attach to serotonin receptors and inhibit them—also referred to as Setrons—are widely prescribed to control vomiting associated with cancer therapies and gastrointestinal diseases. But many of these drugs come with severe side effects. "Many people that take these drugs experience unpleasant symptoms. These side effects have prevented more widespread use," says study lead Sudha Chakrapani, PhD, associate professor of physiology and biophysics at Case Western Reserve University School of Medicine. She suggests designing drugs that target specific conformation of serotonin receptors could help.
"Successful design of safer therapeutics has slowed because there is currently a limited understanding of the structure of the serotonin receptor itself, and what happens after serotonin binds," Chakrapani says. "Our new structure of the serotonin receptor in the resting state has the potential to serve as a structural blueprint to drive targeted drug design and better therapeutic strategies."
The newly revealed structure sets the stage for determining structures of the receptor in the active and drug-bound forms. These discoveries are crucial in painting a complete picture of how the receptor works. Researchers could apply the same technology used in the new study—cryo-electron microscopy—to reveal additional serotonin receptor conformations. The technology earned a 2017 Nobel prize and has already helped researchers resolve 3D structures for proteins central to kidney stones and other ailments. "Cryo-EM" enables researchers to zoom in and see protein structures in atomic detail. The high-powered microscopes take snapshots of proteins in action, and compile them into 3D structural models.
More information: Sandip Basak et al, Cryo-EM structure of 5-HT3A receptor in its resting conformation, Nature Communications (2018). DOI: 10.1038/s41467-018-02997-4
Journal information: Nature Communications
Provided by Case Western Reserve University