Researchers systematize the methods for synthesis of azocine-containing systems
The search for synthetic routes for new drugs plays an extremely important role in contemporary medicinal chemistry. Organic chemists from Russia conducted a systematic study of modern advances in the methods for the synthesis of annulated azocines. The results of this analytical work were published by RUDN University professor Leonid Voskressensky and candidate of chemical sciences Anna Listratova in the journal Synthesis.
Azocines are heterocyclic organic compounds consisting of an eight-membered ring with one nitrogen atom and four double bonds (the simple formula of azocine itself is C7H7N).
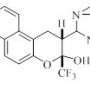
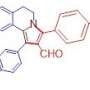
Such systems, partially saturated by hydrogen (hydrogenated or partially hydrogenated azocines) are part of many biologically active compounds, both natural and synthetic. Opioids and alkaloids are among them, for example, cyclazocine or nakadomarin A isolated from a sea sponge and shows high antitumor, antimicrobial, antiinflammatory and antimalarial activity.
However, the biological activity of annulated azocines is poorly studied due to a lack of effective synthesis methods. In this review, all possible ways of synthesis of annulated azocines developed over the last 10 years are considered.
The first and fairly common way of synthesis is ring-expansion reaction: A fragment of the reagent is embedded in the existing cycle, which leads to the formation of an eight-membered cycle. The second method is the intramolecular Heck reaction: a chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of catalyst—to form a substituted alkene.
The third method is a cycloaddition reaction, in which several different linear molecules combine with each other thus forming a cycle. The standard of such addition is the Nobel-winning Diels–Alder reaction, which has become an important method of organic synthesis. In 2009, the first cycloaddition reaction leading to the synthesis of azocines was developed. In the review, the authors consider the further development of such reactions.
The fourth group is the so-called ring-closing metathesis. Robert Grubbs, Richard Schrock and Yves Schowen were awarded the 2005 Nobel Prize in Chemistry for its discovery. In this reaction redistribution of substituents with double bonds takes place. In the case of azocines, the reaction occurs so that an eight-membered ring closes.
The fifth group is a variety of cyclization reactions, which, as a rule, take place in the presence of catalysts. These reactions enabled scientists to the azocine cycle with various aromatic and heteroaromatic rings, such as benzene, naphthalene, indole, etc. And, finally, the sixth group of reactions: microwave- and photo-assisted reactions of the azocine ring cyclization.
In addition, the authors considered other methods of special reactions that do not fit into a particular group, but they are not less effective in obtaining these important heterocycles. They are, for example, cascade and tandem reactions, aldol condensation and thermolysis.
The new work by the chemists from RUDN University actually creates a thesaurus of syntheses of annulated azocines and is intended to improve the situation with the study and application of these substances.
More information: Leonid Voskressensky et al, Recent Advances in the Synthesis of Hydrogenated Azocine-Containing Molecules, Synthesis (2017). DOI: 10.1055/s-0036-1589500
Provided by RUDN University