Scientists achieve arylation of C-H bonds in mild conditions
Carbon-carbon (C-C) bonds make up the skeleton of all organic molecules. However, creating such ubiquitous C-C bonds artificially is still a complicated task. In particular, since several molecules used in medicine, pharmacology and material chemistry contain aryl groups, devising a way to efficiently and selectively introduce this chemical group is a major goal for organic chemists. Currently, most arylation reactions require harsh reaction conditions, including high temperatures and excess additives.
Scientists at the Center for Catalytic Hydrocarbon Functionalization, within the Institute for Basic Science (IBS, South Korea), devised a method to selectively introduce aryl groups into C-H bonds at room temperature. Published in Nature Chemistry, the study also clarifies the details of this reaction, which turned out to be different from the conventional idea.
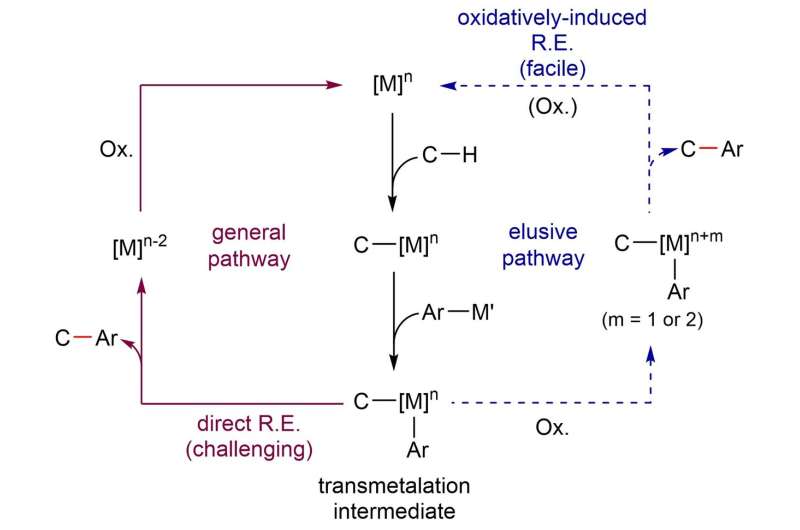
In simple terms, the procedure consists of three main steps. Firstly, the iridium catalyst activates the C-H containing substrate. Secondly, the arylsilane attacks the metal, creating an intermediate molecule. The team crystallized such intermediate and demonstrated that oxidizing the iridium center of the intermediate (third step) is beneficial to achieve a low energy arylation reaction.
The proposed reaction mechanism was verified with electroparamagnetic resonance, cyclic voltametry and computer simulations. "Developing more efficient and environmentally benign oxidation system is our next goal," concludes Kwangmin Shin, first author of the study.
-
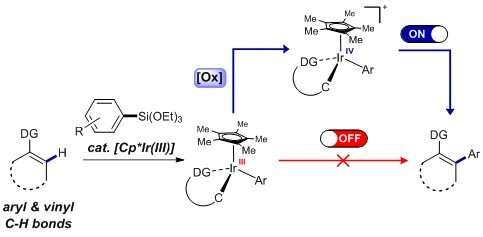
Arylation mechanism proposed by IBS researchers. The reaction proceeds through a novel pathway in which the reductive elimination takes place more easily after the intermediate is selectively oxidized. Credit: Institute for Basic Science -
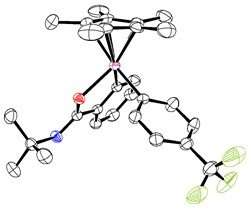
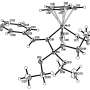
IBS scientists were able to isolate and crystallize the reaction intermediate . Credit: Institute for Basic Science
More information: Kwangmin Shin et al. Iridium-catalysed arylation of C–H bonds enabled by oxidatively induced reductive elimination, Nature Chemistry (2017). DOI: 10.1038/nchem.2900
Journal information: Nature Chemistry
Provided by Institute for Basic Science