Researcher precisely tracks movements of a single catalyst particle
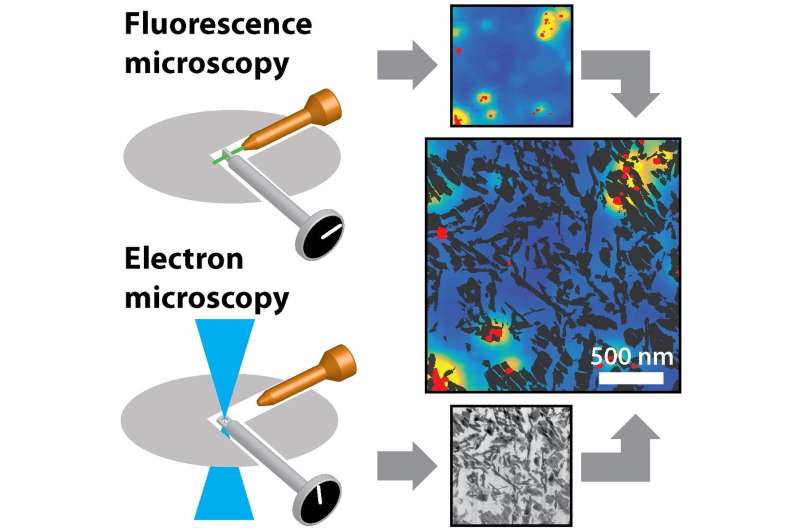
Using fluorescence microscopy, Ph.D. Candidate Frank Hendriks has studied the accessibility, structure and reactivity of individual catalyst particles. His work has resulted in several technological breakthroughs, as well as two important publications. Hendriks will defend his dissertation in the University Hall on 20 December.
"In my first project, I studied a complete catalyst particle with a microscope and used fluorescence in order to follow a single specific type of molecule with a high degree of precision," Hendriks explains. He followed the molecule's movements through the network of pores in a single catalyst particle. "You can really follow an individual molecule," he adds. "That's extremely special, but it's also extremely difficult. This was the first time that the technology was used on a real catalyst, instead of a model system. It shows how complex the catalyst's 'road map' really is. There are many roads that can lead to Rome, or the catalytically active location."
Mountain of information
After two weeks of experiments, Hendriks started working on the resulting data. "That was a whole mountain of information; a total of 60,000 video frames. Gigabytes of data. It was tough to turn all that into a good story." After two years spent analysing the data, Hendriks was able to draw connections between the movement of the molecules and the network of pores in the catalyst particles.
In model systems, the molecules that are observed often display only small variations in speed, because they are located in a simple network of pores. "In the complex network of our catalyst particle, things looked quite a bit different," says Hendriks. "We saw a wide variety of speeds, because the molecules moved through differently sized pores." The results of this study, a collaborative effort by Utrecht University and the University of Leuven, were published last summer in the influential Journal of the American Chemical Society.
Extremely high resolution
In the second half of his Ph.D. studies, Hendriks examined the relationship between the structure of a catalyst and its reactivity. To do so, he used a new method for cutting the catalyst material into thin slices, which allowed him to observe for the first time both the structure and activity of the catalyst at extremely high resolution. The results showed that not all of the zeolites in the catalyst particles are equally active, even though they had similar structures.
In contrast to his first experiment, most of his work involved getting the unique test installation working, rather than analysing the results. "It was a challenge just to be able to conduct measurements. For this experiment, we actually combined two different types of equipment, one of which only works in a vacuum. It took a year before we could get the combination working at all." The analysis of the data for this experiment, which resulted in a publication in the leading journal Angewandte Chemie, took six months to complete.
Hendriks didn't mind that it took him so long to get the test installation working. "Getting something to work is a very concrete puzzle. The fact that it was so difficult to analyse the data from my first experiment was more frustrating, because I didn't know what it would turn up, or if it would even turn up any results at all. With a test installation, you immediately know if it works or not."
More information:
Integrated Transmission Electron and Single-Molecule Fluorescence Microscopy Correlates Reactivity with Ultrastructure in a Single Catalyst Particle
Frank C. Hendriks, Sajjad Mohammadian, Zoran Ristanović, Sam Kalirai, Florian Meirer, Eelco T. C. Vogt, Pieter C.A. Bruijnincx, Hans C. Gerritsen, Bert M. Weckhuysen
Angewandte Chemie, 20 november 2017, DOI: 10.1002/anie.201709723
Single-Molecule Fluorescence Microscopy Reveals Local Diffusion Coefficients in the Pore Network of an Individual Catalyst Particle
Frank C. Hendriks*, Florian Meirer*, Alexey V. Kubarev, Zoran Ristanović*, Maarten B. J. Roeffaers, Eelco T. C. Vogt*, Pieter C.A. Bruijnincx*, and Bert M. Weckhuysen*
Journal of the American Chemical Society, 13 september 2017, DOI: 10.1021/jacs.7b07139
Journal information: Journal of the American Chemical Society , Angewandte Chemie
Provided by Utrecht University Faculty of Science



















