When water met iron deep inside the Earth, did it create conditions for life?
Reservoirs of oxygen-rich iron between the Earth's core and mantle could have played a major role in Earth's history, including the breakup of supercontinents, drastic changes in Earth's atmospheric makeup, and the creation of life, according to recent work from an international research team published in National Science Review.
The team—which includes scientists from Carnegie, Stanford University, the Center for High Pressure Science and Technology Advanced Research in China, and the University of Chicago—probed the chemistry of iron and water under the extreme temperatures and pressures of the Earth's core-mantle boundary.
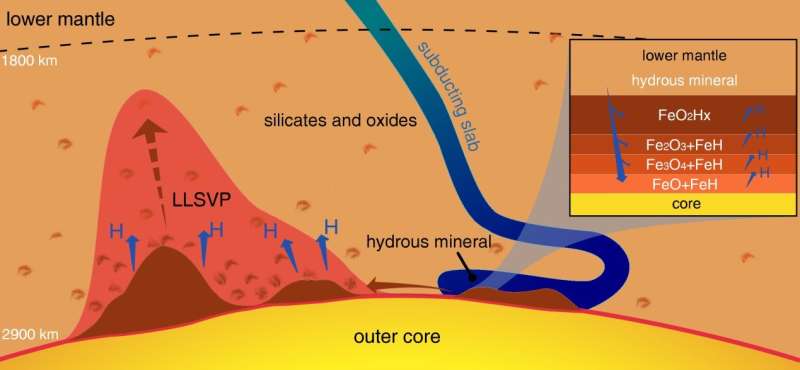
When the action of plate tectonics draws water-containing minerals down deep enough to meet the Earth's iron core, the extreme conditions cause the iron to grab oxygen atoms from the water molecules and set the hydrogen atoms free. The hydrogen escapes to the surface, but the oxygen gets trapped into crystalline iron dioxide, which can only exist under such intense pressures and temperatures.
Using theoretical calculations as well as laboratory experiments to recreate the environment of the core-mantle boundary, the team determined that iron dioxide can be created using a laser-heated diamond anvil cell to put materials under between about 950 and 1 million times normal atmospheric pressure and more than 3,500 degrees Fahrenheit.
"Based on our knowledge of the chemical makeup of the slabs that are drawn into the Earth's deep interior by plate tectonics, we think 300 million tons of water could be carried down to meet iron in the core and generate massive iron dioxide rocks each year," said lead author Ho-kwang "Dave" Mao.
These extremely oxygen-rich solid rocks may accumulate steadily year-by-year above the core, growing into gigantic, continent-like sizes. A geological event that heated up these iron dioxide rocks could cause a massive eruption, suddenly releasing a great deal of oxygen to the surface.
The authors hypothesize that such an oxygen explosion could put a tremendous amount of the gas into the Earth's atmosphere—enough to cause the so-called Great Oxygenation Event, which occurred about 2.5 billion years ago and created our oxygen-rich atmosphere, conditions that kickstarted the rise oxygen-dependent life as we know it.
"This newly discovered high-temperature and intense-pressure water-splitting reaction affects geochemistry from the deep interior to the atmosphere" said Mao. "Many previous theories need to be re-examined now.
Provided by Carnegie Institution for Science