Breakthrough in clean diesel research

A breakthrough in catalysis research by academics at the Universities of St Andrews and Newcastle could lead to the development of clean diesel engine technology and help combat air pollution.
Catalysis is an important process that underpins the chemical industry allowing us to efficiently produce the chemicals that we need. It also allows us to clean-up the pollution that we would otherwise emit into the atmosphere. Catalysts are typically metallic nanoparticles, often platinum group metals that are finely deposited upon a substrate. The activity and durability of the catalyst critically depends upon the interaction of the particles with the substrate.
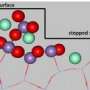
In recent years the team at the University of St Andrews have been exploring metal nanoparticles prepared by exsolution at the surface of perovskite oxides and have shown these structures to enable new dimensions in catalysis and energy conversion and storage technologies owing to their socketed, well-anchored structure.
Now, working closely with researchers at Newcastle University they have demonstrated that contrary to general belief, exsolved particles do not re-dissolve back into the underlying perovskite upon oxidation. Instead, they may remain pinned to their initial locations, and can then undergo further chemical transformations to alter their composition, structure and functionality dramatically, whilst preserving their initial spatial arrangement. This is referred to as 'chemistry at a point'.
The remarkable utility of structures prepared via this concept has been demonstrated in relation to exhaust clean-up from diesel emissions, oxidising CO and NO simultaneously over hundreds of hours of operation. The concept represents a step change in the design of earth-abundant metal catalysts rivalling platinum for reactions of key practical importance, on a weight basis, and also at temperatures relevant to exhaust emissions.
The findings are published today (30 November 2017) in the scientific journal Nature Communications.
Lead academic, Professor John Irvine from the School of Chemistry at St Andrews, said: "This concept 'chemistry at a point' enables the design of compositionally-diverse confined oxide particles with superior stability and catalytic reactivity wide applicability in clean energy processes and environmental remediation.
"In 2015 the Government estimated that exposure to NOx and particulate matter emissions from diesel engines lead to around 52,000 additional deaths in the UK; the findings of the research has far-reaching implications for the future of clean diesel and air pollution."
More information: Dragos Neagu et al. Demonstration of chemistry at a point through restructuring and catalytic activation at anchored nanoparticles, Nature Communications (2017). DOI: 10.1038/s41467-017-01880-y
Journal information: Nature Communications
Provided by University of St Andrews