New target for development of innovative antibiotics
In an article published in Nature Communications on October 3, a group of scientists from Brazil and France describes a new strategy that could be useful to treat infection by drug-resistant pathogens.
The project aims at increasing the efficiency of fighting of the bacillus bacteria—these are rod-shaped or cylindrical bacteria that include several species that cause diseases in humans, such as Escherichia coli, Pseudomonas aeruginosa and Helicobacter pylori.
"Our findings pave the way for the development of antibiotics that have a completely different action mechanism to that of the drugs used today," said Andréa Dessen, coordinator for the project. Dessen does research at the Institute of Structural Biology (IBS) in Grenoble, France, and also at the National Bioscience Laboratory (LNBio) in Campinas, São Paulo State, Brazil.
By improving scientific knowledge of the processes relating to the formation of bacterial cell walls—semi-rigid structures that envelop the entire microorganism and are essential to its survival—the project investigated ways of making bacteria of the bacillus type more vulnerable and incapable of reproducing.
"The cell wall is a mesh like a fishing net made up largely of peptidoglycan, a polymerized blend of sugars and amino acids associated with peptides," Dessen explained. "It protects the bacterium against differences in osmotic pressure and ensures the cell is the right shape. It also contains various factors of virulence [molecules that help bacteria circumvent the defenses of the immune system and infect host cells]."
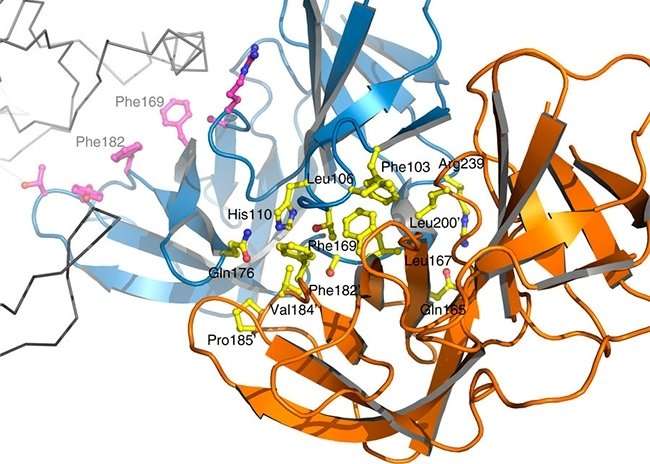
Soon after cell division, certain bacilli proteins need to bind in order to ensure that daughter cells have cell walls with the proper elongated shape. This binding forms a multiprotein complex called the elongasome. The group succeeded for the first time in isolating the central part of the complex formed by proteins PBP2 and MreC and elucidating its three-dimensional structure.
To do this, they used X-ray diffraction crystallography, a technique that consists of crystallizing proteins and observing how the crystal scatters a beam of incident X-rays. "In this way, it was possible to understand how the two molecules interact and plan ways of inhibiting this interaction," Dessen said.
The next step was to engineer mutant versions of MreC with alterations in the amino acids located in the region of the interface with PBP2. In vitro assays showed that the modified protein was no longer able to interact with PBP2 to form the complex.
Strains of the bacterium H. pylori genetically modified to express the mutant protein MreC were produced via collaboration with researchers at France's Pasteur Institute. The group found that when these microorganisms were placed in a culture dish to grow, they were unable to acquire the capsule shape and quickly died. "The alteration made to MreC really did affect cell wall shape," Dessen said. "So the experiment proved the importance of the PBP2-MreC complex to elongation of the wall and survival of bacilli. This knowledge can be used to seek molecules capable of interrupting the interaction between these proteins and thereby kill the bacillus."
In principle, the strategy is effective only against species with elongated cell walls. This group includes Acinetobacter baumannii, which the World Health Organization (WHO) considers one of the most dangerous pathogens today because it has acquired resistance to most available drugs. Another major threat, according to Dessen, is the species Klebsiella pneumoniae, which also has an elongasome. "A woman recently hospitalized in the U.S. died from infection by a strain of K. pneumoniae that's resistant to 26 different antibiotics. The problem of drug-resistant bacteria is serious and hasn't been given proper attention by either governments or the pharmaceutical industry. We can no longer ignore it," Dessen said.
More information: Carlos Contreras-Martel et al, Molecular architecture of the PBP2–MreC core bacterial cell wall synthesis complex, Nature Communications (2017). DOI: 10.1038/s41467-017-00783-2
Journal information: Nature Communications
Provided by FAPESP