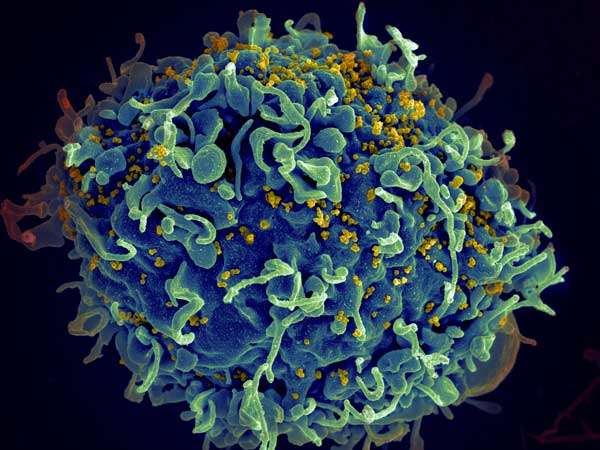
When HIV drugs don't cooperate
The term "synergy" has gained a reputation as an overused buzzword, but it has a quantifiable definition in pharmacology. Two drugs are considered synergistic when their effectiveness when used together is greater than the sum of their effects alone. That is, a drug that is synergistic with another doesn't just perform a beneficial function itself, but makes the second drug perform its function better.
Researchers at Thomas Jefferson University studying combinations of drugs against HIV have discovered why some drugs sometimes act synergistically but sometimes do not. The paper describing their research will be published in the Oct. 6 issue of the Journal of Biological Chemistry.
Second-line HIV drugs, used after first-line treatments have failed, target several different steps in the process by which the virus enters human T cells. Because of the particular steps and proteins they target, two types of these drugs, called co-receptor antagonists and fusion inhibitors, are expected to be synergistic. But multiple previous studies have yielded contradictory results: sometimes these drug classes were indeed strongly synergistic, but sometimes they displayed no synergy at all.
Co-receptor antagonists like maraviroc (marketed under the brand name Selzentry) bind to receptors on host cells known as co-receptors. Fusion inhibitors like enfuvirtide (marketed as Fuzeon), bind to a viral protein called gp41 when it's in a particular transitional phase. To understand why these drugs don't always synergize as expected - and to gain a better understanding of the steps of the HIV infection process - associate professor of biochemistry and molecular biology Michael Root and his then-graduate student Koree Ahn applied different doses of maraviroc and enfuvirtide to cells and viruses with slightly different genetic sequences.
"We found that many different factors are important for [determining] whether there's a synergistic interaction between these two classes of inhibitors or not," Ahn said.
The first factor was the strength of the binding between enfuvirtide and gp41, which could vary depending on mutations in the viral gene that encodes gp41. If the sequence of the gp41 protein was such that enfuvirtide bound to it very tightly, then enfuvirtide and maraviroc acted synergistically. But the weaker the binding, the weaker the synergy between the two drugs.
The implication of this finding is that when virus proteins evolve to avoid binding drugs, it doesn't affect only the efficacy of the drug in question; it also affects how much its effects are "boosted" by other drugs. This is bad news for patients because adding synergistic drugs to a treatment regimen is thought to be a way to combat loss of drug efficacy.
The second factor affecting synergy was the density of co-receptors on host cells, which can vary significantly between patients. "Some [patients] might have very high levels of [co-receptors] on their T-lymphocytes, and those patients would see robust synergy between these two classes of drugs," Root said. "Another individual might have lower levels of co-receptors on the cell surface, and therefore not have as robust synergy, or none at all."
Together, these results suggest that variation in viruses and in patients need to be considered when predicting the efficacy of drug combinations, including newly developed co-receptor antagonists and fusion inhibitors. The paper by Ahn and Root suggests mathematical models for doing just that.
"You need to use these [drugs] with care," Root said. "Drug resistance can emerge with either one, and when resistance emerges you lose that extra benefit of synergy."
More information: Koree W. Ahn et al, Complex interplay of kinetic factors governs the synergistic properties of HIV-1 entry inhibitors, Journal of Biological Chemistry (2017). DOI: 10.1074/jbc.M117.791731
Journal information: Journal of Biological Chemistry