Researchers make headway in desalination technology

Engineers at the University of Illinois have taken a step forward in developing a saltwater desalination process that is potentially cheaper than reverse osmosis and borrows from battery technology. In their study, the researchers are focusing on new materials that could make desalination of brackish waters economically desirable and energy efficient.
The need for practical desalinization technology is rising in the context of global climate change. Coastal regions, where the rise of seawater could encroach upon and contaminate groundwater aquifers, present just one area of concern. As demand for diminishing clean water sources increases, the need for desalination of lower-salinity brackish water from inland and industrial sources will increase, the researchers said.
Illinois mechanical science and engineering professor Kyle Smith and his co-authors have published a study demonstrating the viability of this batterylike technology in the journal Electrochimica Acta.
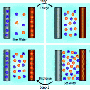
In a previous study, Smith and his co-authors used theoretical modeling to show that technology used in sodium-ion batteries may efficiently desalinate seawater. Their theory states that by using electrodes that contain sodium and chloride ions, salt is drawn out and held in a chamber separate from the purified water.
"In our new study, we constructed and experimented with a batterylike device that uses electrodes made from a different material. That material can remove from brackish water not only sodium ions but also potassium, calcium, magnesium and others," Smith said. "This is important because salt and brackish waters do not contain just sodium chloride. It is often in a mix with other salts like potassium, calcium and manganese chloride."
The new material is a chemical analog to the compound Prussian blue - the intense pigment used in ink for blueprints. It works by taking and holding positively charged ions like sodium within its crystal structure, Smith said.
"The competition between the rate of diffusion of the positively charged ion within the crystal structure and the volume at which the ions can be stored creates a traplike structure," Smith said. "They go in easily but can't get out."
There are other materials that can secure positive ions, but the Prussian blue analog has an additional benefit - it is potentially very cheap to source.
"To make a technology like this be economically feasible, it needs to be cheap and, ideally, have some value-added benefit," Smith said. "By showing that our device works well with lower-salinity waters, the door for use with inland brackish waters and possibly industrial wastewater has opened."
Smith and his co-authors show that the amount of salt removal is sufficient to demonstrate their concept using brackish water. However, further research is needed to determine how the removal of salts from higher-salinity seawater and wastewater will impact that energy efficiency.
More information: Slawomir Porada et al, Nickel Hexacyanoferrate Electrodes for Continuous Cation Intercalation Desalination of Brackish Water, Electrochimica Acta (2017). DOI: 10.1016/j.electacta.2017.09.137
Provided by University of Illinois at Urbana-Champaign