Handful of molecular machines run the biochemical oscillator that sets the timing of many bodily processes
Scientists have long known that circadian clocks—biochemical oscillators that control physiology, metabolism and behavior on a roughly 24-hour cycle—are present in all forms of life, including animals, plants, fungi and some types of bacteria. However, the molecular mechanisms that "run" these systems remain largely unknown.
In a study published Sept. 7 in Molecular Cell, a team led by Harvard Medical School researcher Charles Weitz shows that a set of core clock proteins organize themselves into a handful of molecular machines that control the precise workings of circadian rhythms.
Providing the first structural glimpse of the clock's machinery, the results offer a starting point for explaining how circadian clocks run and an understanding of the variety of conditions that can develop—including sleep disorders, metabolic aberrations and cancer—when something in the clock machinery goes awry.
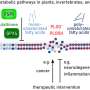
In the late 1990s, Weitz, the Robert Henry Pfeiffer Professor of Neurobiology at Harvard Medical School, and researchers from other labs discovered several key proteins involved in the clock system. These include three different period proteins (PER), two different cryptochrome proteins (CRY), and casein kinase-1 (CK1). When these proteins accumulate inside cells and enter the cell nucleus, they bind to a protein called CLOCK-BMAL1 that is attached to DNA responsible for making more PER and CRY. The influx and accumulation of these proteins inside the nucleus effectively shut down the production of PER and CRY. However, when the levels of PER and CRY drop, the CLOCK-BMAL1 can once again resume work unhindered so that the DNA responsible for making PER and CRY can do its job.
The completion of this feedback loop—production of PER and CRY, their attachment to CLOCK-BMAL1, shutting down PER and CRY production so that it can start over again—takes about 24 hours, Weitz explains. The traditional view, he adds, is that these proteins enter the cell nucleus independently or in small groups to do different jobs. The Weitz team findings revealed otherwise.
To figure out precisely how these proteins might run the clock, Weitz and colleagues used a laboratory technique that selectively pulled out proteins from the nuclei of mouse cells at the peak of PER and CRY negative feedback. Their findings turned up a single large protein complex that incorporated each of the six important clock proteins: the three PERs, two CRYs, and CK1, along with about thirty other accessory proteins. Additionally, the protein complex, which electron microscopy showed is quasi-spherical, was associated with CLOCK-BMAL1, the experiments showed.
Although their initial experiments were done in mouse livers—large organs with a strong concentration of different proteins—experiments in other tissues, including kidney and brain, detected the presence of the same large protein complex. The results suggest that this complex, which the researchers named the PER complex, is universal in tissues throughout the body. They also suggest that the six key clock proteins probably don't operate individually; instead, they seem to organize themselves to work in concert to run the circadian clock's negative feedback loop.
To determine when this organization happens, the researchers looked for the presence of the six main clock proteins in the cytoplasm, the gooey liquid inside a cell that surrounds the nucleus and other organelles. There, they found four other complexes composed of different groups of the six proteins—one with all six, named the upper complex—and three others missing one or more of these key proteins. The researchers hypothesized that these complexes were in various states of assembly, but that the six key proteins entered the nucleus as a group.
The upper complex also had a seventh protein called GAPVD1, known from other studies to help shepherd chemicals to different locations inside cells. Although the role of GAPVD1 in the circadian clock remains somewhat unclear, Weitz said, experiments in which he and his colleagues trimmed this protein out of the upper complex caused disruption in circadian cycle—an observation that suggests GAPVD1 plays a key role in the clock.
Weitz cautions that the precise orchestration performed by this constellation of proteins in running the body's clock remains yet to be teased out. However, he said, learning more about how these proteins interact has given researchers a clearer clue into inner workings of the system overall.
"The circadian clock is a very deep timing system that controls a large part of the physiology and behavior in all cells in the body to shape multiple processes," Weitz said. "The more we learn about it, the more links we'll get to certain kinds of disease states that aren't easily amenable to treatment today. Now that we understand how these molecular machines are built, we can start asking questions about how they work."
Journal information: Molecular Cell
Provided by Harvard Medical School