September 29, 2017 report
Delignification of wood samples using p-toluenesulfonic acid as a recyclable hydrotrope
(Phys.org)—Lignin is an important component of the cell wall in plant cells and accounts for rigid structures, such as tree bark. It is an organic polymer that is insoluble in water, and encases cellulose fibers via attachment to various polysaccharides that are collectively known as hemicelluloses. Part of the process of making paper is to remove lignin, or delignification. Additionally, there is much interest in finding simple, environmentally friendly ways to remove lignin to access cellulose for biofuels and to use as a bioproduct. However, delignification processes tend to involve harsh conditions, such as high temperatures and pressure, and require caustic reagents.
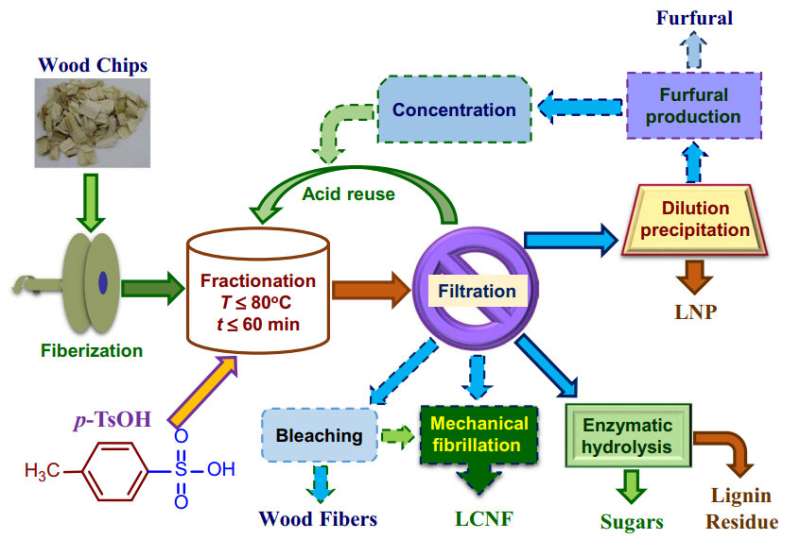
Several researchers hailing from institutions in China, Finland, and the United States, led by Dr. Junyong Zhu of the USDA Forest Products Laboratory in Madison, Wisconsin, have demonstrated that p-toluenesulfonic acid serves as a good hydrotrope for the near-complete solubilization of wood lignin at temperatures at or below eighty degrees. Furthermore, this process takes less than half the time required in typical delignification processes. This hydrotrope left much of the lignin structure intact, allowed for the separation of other sugars from wood sample, and demonstrated reusability. Their study appears in Science Advances.
One way to recover pure polysaccharides from linocellulosic biomass is to separate lignin. This is best achieved in an aqueous system rather than in solvents due to solvent recovery costs and environmental concerns. Hydrotropes are a good option for solubilizing the normally insoluble lignin components in water, while leaving water insoluble sugars behind. P-toluenesulfonic acid, or p-TsOH, has a hydrophilic portion (sulfonic acid) and a hydrophobic portion (toluene), making it a good candidate for an effective hydrotrope.
After testing Wiley-milled poplar NE22 particles with p-TsOH at various concentrations, temperatures, and reaction times, the authors found that p-TsOH at concentrations high enough to form aggregate clusters and at temperatures at or below 80oC resulted solubilized 90 % of the lignin in the sample, making two fractions. One solid fraction contained mainly cellulose that was water insoluble and can be used to produce cellulose nanomaterials, or fibers, or monomeric sugars through enzymes for biofuel or biochemical products.
The other liquid fraction contained solubilized lignin and some hemicellulose sugars, that can be converted into furfural by the p-TsOH in the spent liquor.
The authors were able to retrieve solid lignin from the dissolved fraction via dilution and precipitation. Because p-TsOH is a hydrotrope, it must form aggregates to solubilize lignin. This means that there is a minimal concentration of p-TsOH needed to form aggregates, known as the minimum hydrotrope concentration, or MHC. The authors found that the MHC for p-TsOH is about 11.5 wt%. After wood fractionation, the fraction with lignin in it can be diluted to below the MHC to precipitate lignin.
The authors then examined the lignin precipitate using atomic force microscopy. They found that the gentler procedure for solubilizing lignin used in this study resulted in a range of lignin aggregate sizes (100 nm to 1.5 μm). This is of interest for applications in developing biodegradable materials.
To understand how different concentrations of p-TsOH and temperatures affected solubilization, the authors analyzed the poplar fractions with 2D NMR and compared their fractions to poplar whole-cell walls. They then used these results to optimize concentration and temperature conditions to allow for separation of polysaccharides from the cell wall. Based on their NMR results, the best concentration of p-TsOH is 70 wt%. The temperature can be lowered from 80oC to 65oC and still result in lignin separation from polysaccharides within the cell wall.
Finally, the authors conducted a preliminary test that demonstrated p-TsOH could be removed from the spent liquor through re-crystallization and re-used. Their findings showed negligible differences between the amount of lignin that was solubilized in the first cycle compared to the second cycle showing that p-TsOH likely has good recyclability. Additional tests will need to be done to quantify the amount of p-TsOH consumed.
Overall, this research demonstrates that p-toluenesulfonic acid is a viable candidate for large-scale delignification processes because it is recyclable, uses relatively mild conditions, and solubilizes almost all of the lignin in wood samples.
More information: Liheng Chen et al. Rapid and near-complete dissolution of wood lignin at ≤80°C by a recyclable acid hydrotrope, Science Advances (2017). DOI: 10.1126/sciadv.1701735
Abstract
We report the discovery of the hydrotropic properties of a recyclable aromatic acid, p-toluenesulfonic acid (p-TsOH), for potentially low-cost and efficient fractionation of wood through rapid and near-complete dissolution of lignin. Approximately 90% of poplar wood (NE222) lignin can be dissolved at 80°C in 20 min. Equivalent delignification using known hydrotropes, such as aromatic salts, can be achieved only at 150°C or higher for more than 10 hours or at 150°C for 2 hours with alkaline pulping. p-TsOH fractionated wood into two fractions: (i) a primarily cellulose-rich water-insoluble solid fraction that can be used for the production of high-value building blocks, such as dissolving pulp fibers, lignocellulosic nanomaterials, and/or sugars through subsequent enzymatic hydrolysis; and (ii) a spent acid liquor stream containing mainly dissolved lignin that can be easily precipitated as lignin nanoparticles by diluting the spent acid liquor to below the minimal hydrotrope concentration. Our nuclear magnetic resonance analyses of the dissolved lignin revealed that p-TsOH can depolymerize lignin via ether bond cleavage and can separate carbohydrate-free lignin from the wood. p-TsOH has a relatively low water solubility, which can facilitate efficient recovery using commercially proven crystallization technology by cooling the concentrated spent acid solution to ambient temperatures to achieve environmental sustainability through recycling of p-TsOH.
Journal information: Science Advances
© 2017 Phys.org


















