Researchers crack solvent mixtures puzzle
Chemists working at the University of Amsterdam's (UvA) Sustainable Chemistry research priority area have collaborated with the Solvay Lab of the Future in Bordeaux to develop a practical toolbox for predicting the solubility of small molecules in different solvents. These tools are available open-access and free of charge, and can enhance solvent selection and formulations of many industrial products.
Solvents are extremely important for many industrial sectors. Often, in the formulation of a chemical product the solvent makes the bulk of the entity. It is also crucial for the product's function. For instance, with the right solvent formulation, pesticides stay longer on leaves after rain, paints and inks dry faster, and cosmetics are applied more easily. Knowing the solubility of molecules is therefore essential for product development.
The problem with small molecules
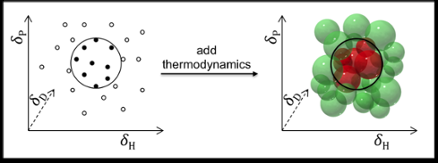
The prediction of solubility is usually done using the so-called Hansen Solubility Parameters: dispersion (D), polar interactions (P), and hydrogen bonding (H). The coatings and polymers industry, for example, obtains excellent results using these parameters for predicting the solubility of polymers.
In principle, Hansen parameters can also be used to find solvents for smaller molecules like drugs and cosmetics. But there the predictions are not as satisfactory, for two reasons: One, because drugs and cosmetics typically have more varied functional groups; and two, because the original Hansen parameters exclude thermodynamic considerations regarding mixing, melting and dissolution. This is acceptable for polymers (where the thermodynamics cancel out) but not for small molecules.
Dr Manuel Louwerse and Prof. Gadi Rothenberg, working together with the team of Dr Bernard Roux at Solvay, have now improved Hansen's model and adapted it to handle small-molecule solutes by including the thermodynamics of mixing, melting and dissolution. The improvements are based on a better description of both the entropy and the enthalpy terms. When a compound dissolves, molecules leave the crystal and mix into the solvent. This increases the entropy, but usually costs some enthalpy. The key issue here is that the amount of entropy gained by mixing determines how much enthalpy can be lost while keeping a negative ∆G (in other words, maintaining the driving force for the dissolution). Since the entropy effect depends on the concentration, the temperature, and the size of the molecules, these should all be included.
Another improvement was made by splitting the contributions of electron donors and acceptors among the solvent and solute. This is especially important for cases like hydrogen bonding, which is relevant to many solvents and solutes. The mantra 'like dissolves like' is too simplistic here. Hydrogen bonds form between donors and acceptors, so one needs donors to dissolve acceptors, and vice versa. By splitting the donor and acceptor contributions of each solvent and solute, the UvA team obtained more accurate models.
The new models are much better at predicting the solubility of small molecules in solvents and solvent blends. Tests on a large industrial dataset of 15 different solutes and 48 solvents and their blends at the Solvay Lab of the Future showed that fit qualities improved from 0.89 to 0.97. The percentage of correct predictions rose from 54% to 78%. Since just guessing would already yield 50% correct predictions, this is a serious improvement. Another important advantage is that the new model enables predictions at extrapolated temperatures.
The results and the models are published as an open-access paper in the peer-reviewed international journal ChemPhysChem. The paper has already raised many comments, and the improvements are now being incorporated into a newer version of the HSPiP software.
While most of the actual industrial formulation data is confidential, the joint team has published open-access the full description of the theory and the models. They also included the full and annotated Matlab routines in the supporting information, enabling everyone to use these new tools for designing new solvent mixtures and formulations.
Prof. Rothenberg sees the publishing of tools as the key to successful public-private partnerships between industry and academia. 'Industrial partners need to keep their data confidential, but most of them realise that open-access publishing of the methods and tools creates goodwill and enables further developments by both collaborators and competitors. By sharing methods and tools, companies can benefit from each other's knowledge without sacrificing data.'
More information: Manuel J Louwerse et al. Revisiting Hansen solubility parameters by including thermodynamics, ChemPhysChem (2017). DOI: 10.1002/cphc.201700408
Journal information: ChemPhysChem
Provided by University of Amsterdam