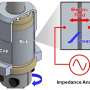
Scientists analysed how a special treatment improves cheap metal oxide photoelectrodes
The fossil fuel age is bound to end, for several strong reasons. As an alternative to fossil fuels, hydrogen seems very attractive. The gas has a huge energy density, it can be stored or processed further, e. g. to methane, or directly provide clean electricity via a fuel cell. If it is produced using sunlight alone, hydrogen is completely renewable with zero carbon emissions.
Artificial leafs
Similar to a process in natural photosynthesis, sunlight can also be used in "artificial leafs" to split water into oxygen and hydrogen. Artificial leafs combine photoactive semiconducting materials and can reach efficiencies beyond 15 %. However, those record efficiencies were obtained using expensive systems, which also tend to decompose in aqueous solutions. For successful commercialization costs need to go down and stability needs to increase.
Good candidates with one disadvantage
Complex metal oxide semiconductors are good candidates for artificial leafs since they are relatively cheap and stable in aqueous solutions. Scientists from HZB-Institute for Solar Fuels focus their research on these materials. Until now, photoelectrodes based on metal oxides have shown moderate efficiencies (only < 8 %). One reason is their poor charge carrier (electron and/or hole) mobility, which is up to 100.000 times lower than in classical semiconductors such as gallium arsenide or silicon. "What is worse is the fact that charge carriers in metal oxides often have really short life spans of nanoseconds or even picoseconds. Many of them disappear before they can contribute to water splitting", Dr. Fatwa Abdi, expert at HZB-Institute for Solar Fuels points out.
Heat treatment with hydrogen
One option to overcome this limitation is a heat treatment under hydrogen atmosphere of the metal oxide layers after deposition. Fatwa Abdi and his colleagues have now investigated how this treatment influences life spans, transport properties and defects in one of the most promising metal oxide photoelectrodes, bismuth vanadate (BiVO4).
Life spans of charge carriers doubled
Time-resolved conductivity measurements revealed that electrons and holes live more than twice as long in the bulk of the hydrogen-treated BiVO4 as compared to the pristine BiVO4. As a result, the overall photocurrent under sunlight is largely improved. Further measurements at Dresden and theoretical calculations by KAUST-colleagues in Saudi Arabia provided evidence that the presence of hydrogen in the metal oxide reduces or deactivates point defects in the bulk of BiVO4. "Our results show that hydrogen treatment leads to less traps for charge carriers and less opportunities to recombine or getting lost. So more charge carriers survive for longer and may contribute to water splitting", Abdi explains.
More information: Ji-Wook Jang et al, Enhancing Charge Carrier Lifetime in Metal Oxide Photoelectrodes through Mild Hydrogen Treatment, Advanced Energy Materials (2017). DOI: 10.1002/aenm.201701536
Journal information: Advanced Energy Materials
Provided by Helmholtz Association of German Research Centres