It's never too cold for quantum
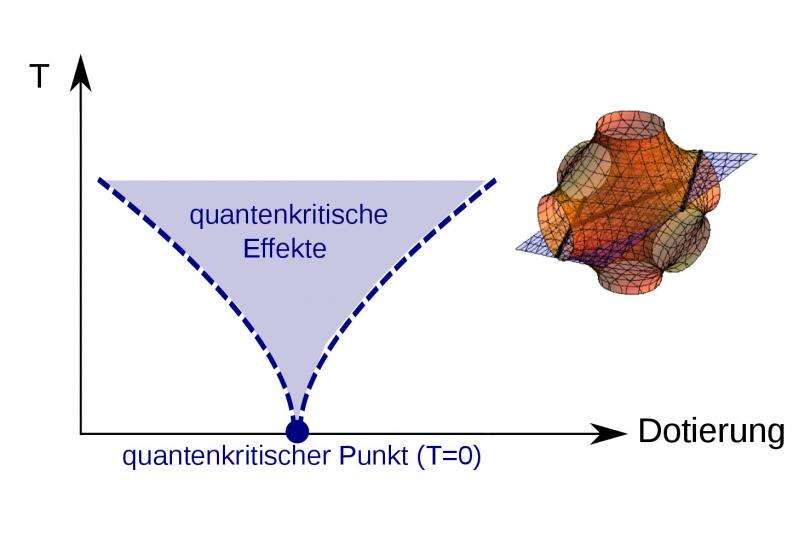
The peculiar characteristics demonstrated by quantum critical points at absolute zero remain one of the great unsolved mysteries of science.
Normally, there needs to be a change in temperature in order to see a phase transition: a liquid gets cold, it freezes; a metal heats up, it loses its magnetic properties. But there are some phase transitions in which the temperature cannot change, because they occur right at absolute zero. The quantum critical points where such transitions take place have been the subject of intensive research for many years, yet they are still hugely puzzling for quantum physicists.
Until now, for example, there has been no comprehensive theoretical model for the high-temperature superconductivity that is suspected to be closely related to quantum critical points – although such a model could generate a lot of useful technical applications. Thomas Schäfer, Karsten Held and Alessandro Toschi of the Institute of Solid State Physics at TU Wien are working towards a better understanding of these phenomena, publishing their new ideas on this field in the journal Physical Review Letters.
Fluctuations: if it can shake, it will shake
"Thermal fluctuations are usually responsible for phase transitions," explains Thomas Schäfer. "Individual particles start to shake or rotate, for instance, completely at random. The higher the temperature, the more pronounced these fluctuations become, which can lead to a phase transition – causing a solid to melt, for example."
As you reduce the temperature, the particles move around less and less, until they reach absolute zero, at which point they should no longer move at all. So, one could assume that total calm will have been restored at absolute zero, as nothing is able to change any more ... but it is not quite as simple as that.
"Quantum physics states that it is impossible for a particle to be fully at rest in a specific location," says Alessandro Toschi. "Heisenberg's uncertainty principle tells us that position and momentum cannot be ascertained with total precision. Therefore, a particle's position and momentum can still change at absolute zero, even if classic thermal fluctuations are no longer present. These changes are known as quantum fluctuations."
So, when it is too cold for classic shaking movements, quantum physics ensures that physically interesting things can still happen. And that is exactly why phase transitions at absolute zero are so endlessly fascinating.
Momentum and energy
"What is crucial for the particles' behaviour is how their momentum relates to energy," says Thomas Schäfer. For a ball thrown through the air, the correlation is simple: the greater the momentum, the greater the kinetic energy. The energy increases as the square of momentum. But for particles in a solid, this relationship is much more complicated, and can look very different, depending on the direction in which the particle is moving. Therefore, this connection is modelled using 'Fermi surfaces', which are able to take on complex three-dimensional shapes.
"Until now, it was thought the shape of these Fermi surfaces was not significant in terms of quantum phase transitions," says Karsten Held. "We have been able to show that is not the case. Only if you take the shape into account can you accurately calculate certain physical effects – for example, the way in which a material's magnetic properties will change as it approaches absolute zero."
Now the researchers hope to use this new tool to better describe quantum critical materials – and maybe shed light on some of the great mysteries that materials science has been working so hard to solve for so many years.
More information: T. Schäfer et al. Interplay of Correlations and Kohn Anomalies in Three Dimensions: Quantum Criticality with a Twist, Physical Review Letters (2017). DOI: 10.1103/PhysRevLett.119.046402
Journal information: Physical Review Letters
Provided by Vienna University of Technology