June 23, 2017 report
Energetic cost of the entatic state of cytochrome c quantified
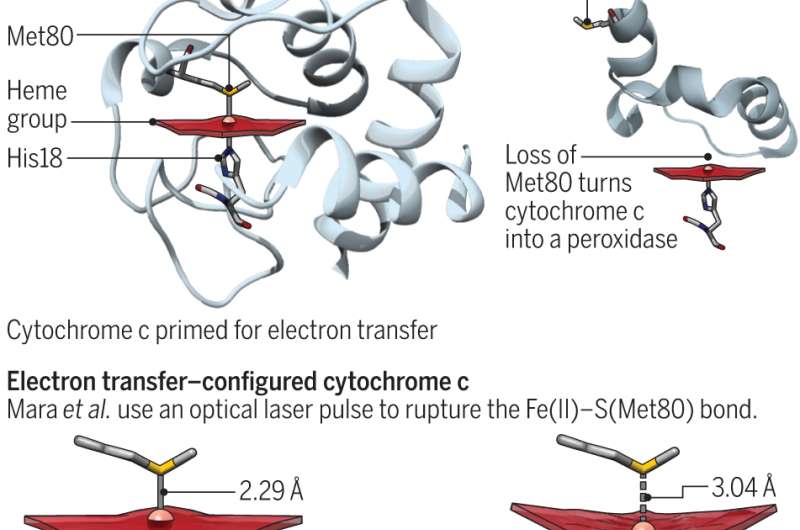
(Phys.org)—A team of researchers at Stanford University has used ultrafast x-ray absorption and emission spectroscopy to quantify the entatic state of cytochrome c. In their paper published in the journal Science, the group outlines their procedure and what they learned. Kara Bren and Emma Raven with the University of Rochester and University of Leicester respectively offer a Perspective piece on the work done by the team in the same journal issue, and outline some of the implications regarding the role that the protein plays in cell life and death.
Cytochrome c is a protein that exists in many plants, animals and unicellular organisms. In humans, its main purposes are ferrying electrons in mitochondria and assisting with apoptosis (normal cell death and the processes surrounding it.) These two functions have been shown in prior efforts to rely on the position of methionine residue. When sulfur works with iron, the protein is ready to transfer electrons. Otherwise, it engages in peroxidase activities. In this new effort, the researchers sought to better understand the energetics of the protein by probing the iron and sulfur bond. Entatic states, Bren and Raven point out, are very important in bioinorganic chemistry—it actually translates to something that is stretched when subjected to tension.
To better understand the bond between the two elements, the researchers temporarily forced them apart using a Linac Coherent Light Source X-ray free electron laser and then timed how long it took the two components to reform using iron X-ray emission spectroscopy. They found that the environment in which they existed boosted bond strength by four kilocalories per mole, which was enough to allow the protein to toggle between its functional states and to quantify the energy cost of the entatic state.
As Bren and Raven note, the results of the study have implications regarding the role that cytochrome plays in respiration, which they relate to living and apoptosis, which they relate to death. To promote continued living the protein helps to maintain a certain reduction potential. For apoptosis, the entatic state is disrupted allowing peroxidase activity to be enhanced.
More information: Metalloprotein entatic control of ligand-metal bonds quantified by ultrafast x-ray spectroscopy, Science (2017). science.sciencemag.org/cgi/doi … 1126/science.aam6203
Abstract
The multifunctional protein cytochrome c (cyt c) plays key roles in electron transport and apoptosis, switching function by modulating bonding between a heme iron and the sulfur in a methionine residue. This Fe–S(Met) bond is too weak to persist in the absence of protein constraints. We ruptured the bond in ferrous cyt c using an optical laser pulse and monitored the bond reformation within the protein active site using ultrafast x-ray pulses from an x-ray free-electron laser, determining that the Fe–S(Met) bond enthalpy is ~4 kcal/mol stronger than in the absence of protein constraints. The 4 kcal/mol is comparable with calculations of stabilization effects in other systems, demonstrating how biological systems use an entatic state for modest yet accessible energetics to modulate chemical function.
Journal information: Science
© 2017 Phys.org