May 9, 2017 feature
Scientists solve 400-year-old mystery of Prince Rupert's drops
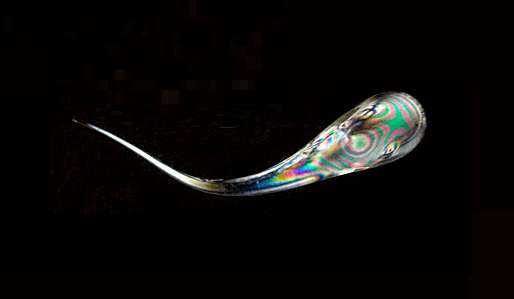
(Phys.org)—Researchers have finally answered a question that has stumped scientists since the early 1600s: Why are the heads of tadpole-shaped pieces of glass called "Prince Rupert's drops" so strong?
In the 17th century, Prince Rupert from Germany brought some of these glass drops to England's King Charles II, who was intrigued by their unusual properties. While the head of the drop is so strong that it can withstand the impact of a hammer, the tail is so fragile that bending it with your fingers will not only break the tail, but cause the entire droplet to instantly disintegrate into a fine powder.
Prince Rupert's drops are easily made by dropping red hot blobs of molten glass into water. Although researchers have tried to understand what causes the unusual properties of these drops for many years, it was not until recently that modern technology has allowed researchers to thoroughly investigate them.
In 1994, S. Chandrasekar at Purdue University and M. M. Chaudhri at the University of Cambridge used high-speed framing photography to observe the drop-shattering process. From their experiments, they concluded that the surface of each drop experiences highly compressive stresses, while the interior experiences high tension forces. So the drop is in a state of unstable equilibrium, which can be easily disturbed by breaking the tail.
One open question, however, is how the stresses are distributed throughout a Prince Rupert's drop. Understanding the stress distribution would help to more fully explain why the heads of these drops are so strong.
To do this, Chandrasekar and Chaudhri began collaborating with Hillar Aben, a professor at Tallinn University of Technology in Estonia. Aben specializes in determining residual stresses in transparent three-dimensional objects, such as Prince Rupert's drops.
In the new study published in Applied Physics Letters, Aben, Chandrasekar, Chaudhri, and their coauthors have investigated the stress distribution in Prince Rupert's drops using a transmission polariscope, which is a type of microscope that measures the birefringence in an axi-symmetrical transparent object, such as a Prince Rupert's drop.
In their experiments, the researchers suspended a Prince Rupert's drop in a clear liquid, and then illuminated the drop with a red LED. Using the polariscope, the researchers measured the optical retardation of the light as it traveled through the glass drop, and then used the data to construct the stress distribution throughout the entire drop.

The results showed that the heads of the drops have a much higher surface compressive stress than previously thought—up to 700 megapascals, which is nearly 7,000 times atmospheric pressure. This surface compressive layer is also thin, about 10% of the diameter of the head of a drop.
As the researchers explain, these values give the droplet heads a very high fracture strength. In order to break a droplet, it's necessary to create a crack that enters the interior tension zone in the drop. Since cracks on the surface tend to grow parallel to the surface, they cannot enter the tension zone. Instead, the easiest way to break a drop is to disturb the tail, since a disturbance in this location allows cracks to enter the tension zone.
Overall, the researchers believe that the results finally explain the great strength of Prince Rupert's drops.
"The work has fully explained why the head of a drop is so strong," Chaudhri told Phys.org. "I believe we have now solved most of the main aspects of this area. However, new questions may emerge unexpectedly."
More information: H. Aben et al. "On the extraordinary strength of Prince Rupert's drops." Applied Physics Letters. DOI: 10.1063/1.4971339
Journal information: Applied Physics Letters
© 2017 Phys.org