Scientists decipher the multi-domain, full-length structure of the human smoothened receptor
A team of scientists led by the iHuman Institute of ShanghaiTech University in collaboration with Fudan University has determined the high-resolution crystal structure of the multi-domain human smoothened receptor. The results illustrate the allosteric domain-domain interactions within the receptor, and their role in smoothened activation. These new findings are published on May 17th, 2017 in Nature Communications, titled "Crystal structure of a multi-domain human smoothened receptor in complex with a super stabilizing ligand," by Zhang X-J et al.
As a central player in the Hedgehog signaling pathway involved in embryonic development and tumorigenesis, the smoothened receptor (SMO) has been long-sought after as a drug target for numerous cancers. Drug resistance however has been an issue due to mutations in SMO. "Development of next-generation anti-SMO drugs will be facilitated by understanding the multi-domain arrangement in the SMO structure", said Fei Xu, Assistant Professor at iHuman Institute, ShanghaiTech University, and the lead corresponding author of this paper. "This structure will allow us to identify potentially new ligand binding sites and signaling mechanisms."
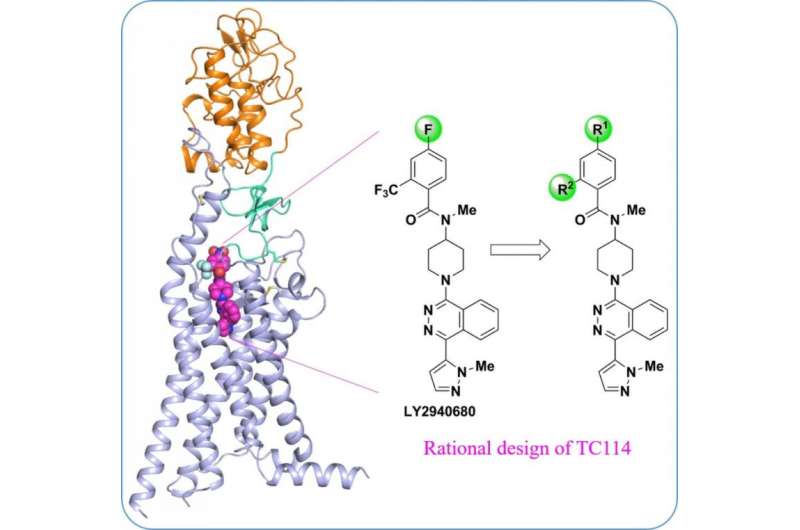
"To stabilize the multi-domain human SMO protein, we designed a series of chemical tool compounds", said Houchao Tao, Research Associate Professor at iHuman Institute. "TC114 is a probe that significantly stabilizes and locks the receptor into a single conformation." With further optimization, PhD student Xianjun Zhang solved the multi-domain SMO structure bound to TC114 at 2.9 angstrom using x-ray free electron laser. "This structure reveals the hinge domain that may play important modulating roles in connecting the domains, providing hints for the development of new modulators targeting this region", said Xianjun Zhang.
"This is beautiful teamwork", said Raymond Stevens, Director of iHuman Institute, ShanghaiTech University, "chemistry and biology are bridged together in this science to understand the structure and function of this complex multi-domain receptor. The crystal structure, in turn, opens new avenues for drug discovery."
More information: Xianjun Zhang et al. Crystal structure of a multi-domain human smoothened receptor in complex with a super stabilizing ligand, Nature Communications (2017). DOI: 10.1038/ncomms15383
Journal information: Nature Communications
Provided by ShanghaiTech University