How enzymes communicate
The enzymes nitric oxide (NO) synthase (NOS1) and protein kinase C (PKC) play an important role in a variety of signal transfer processes in neurons of the brain, as well as in many cell types of other organs. Together with Prof. Dr. Bernd Fakler at the Institute of Physiology at the University of Freiburg, the scientists Dr. Cristina Constantin and Dr. Catrin Müller have shown for the first time that enzymes can be activated under physiological conditions through sole electrical stimulation of the cell membrane. Protein super complexes that rapidly transform electrical signals at the cell membrane into chemical signal processes inside the cell emerge through direct structural interaction of both enzymes with voltage-gated calcium channels. The researchers have presented their work in the current issue of the scientific journal Proceedings of the National Academy of Sciences (PNAS).
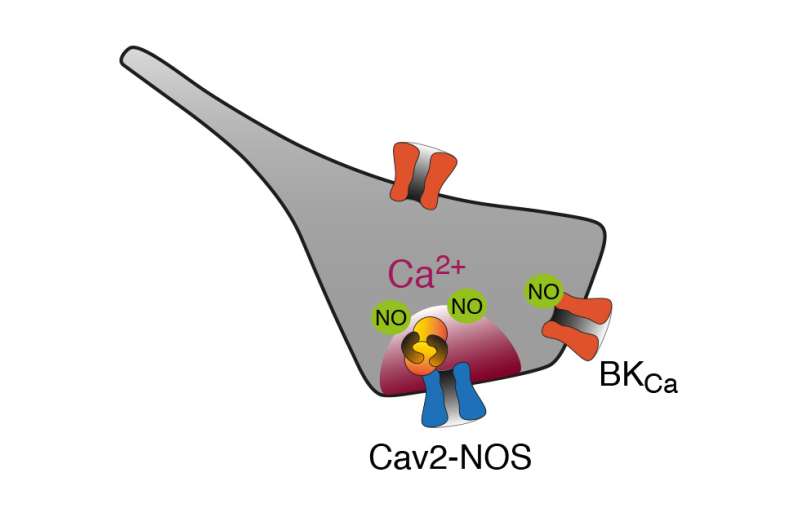
The Fakler group has previously shown that both calcium-dependent enzymes NOS1 and PKC are components of the protein nano-environment of certain voltage-gated calcium channels (Cav2-channels) in the brain. As yet, however, it was not know how these enzymes communicate with the calcium channels. The research group has now found that both enzymes are integrated into protein super complex with Cav2 channels. Within such Cav2-NOS1/PKC complexes NOS1 or PKC are anchored at the cytoplasmic side of the cell membrane and are placed at in the immediate vicinity of the channel pore. Upon excitation of the cell membrane, the Cav2 channels open and deliver calcium ions to the cell cytoplasm, where they bind to both enzymes. Calcium binding activates the enzymes, which subsequently produce the diffusible second messengers NO or phosphorylate cytoplasmic target proteins.
Due to the proximity between channel and enzyme, electrical stimulations of less than a millisecond duration are required for effective electro-chemical coupling. The latter becomes maximal when the cell, instead of being stimulated by individual impulses, fires action potentials with a frequency of one hertz or more.
The Cav2-enzyme super complexes not only guarantee an ultrafast and reliable electro-chemical coupling. They also ensure that signal transduction remains locally restricted, that is, within an area less than a few nanometers around the Cav2 channels. This local restriction guarantees that the enzymes only initiate specific cellular processes, while other calcium signalling pathways, including cell death, are prevented. In addition, the researchers' experiments highlighted the physiological mechanism for activation of NOS1 and PKC thus presenting an alternative to the widely used synthetic activators, such as NO donors or diacylglycerols.
Bernd Fakler is the director of Department II of the Institute of Physiology and area coordinator of the Cluster of Excellence BIOSS Centre for Biological Signalling Studies at the University of Freiburg.
More information: Cristina E. Constantin et al, Identification of Cav2–PKCβ and Cav2–NOS1 complexes as entities for ultrafast electrochemical coupling, Proceedings of the National Academy of Sciences (2017). DOI: 10.1073/pnas.1616394114
Journal information: Proceedings of the National Academy of Sciences
Provided by University of Freiburg