Scientists reveal open-ringed structure of Cdt1-Mcm2-7 complex
Scientists from the Hong Kong University of Science & Technology(HKUST) and Tsinghua University have revealed the open-ringed structure of the Cdt1-Mcm2-7 complex as a precursor of the MCM double hexamer (DH).
Their findings were published online on Feb 13 in Nature Structural Biology and Molecular Biology.
Every cell division requires the prior complete duplication of the genetic DNA so that each daughter cell inherits an exact copy from the mother cell. Replication of DNA is carried out by the DNA replication machine known as the Replisome. The Replisome is a complex assembly of engines that is intricately built to allow the machine to separate the two strands of DNA and each strand to serve as a template for the synthesis of two daughter DNAs. In order to understand the mechanism for DNA unwinding, it is important to know the structure of the core component of the engine or helicase that drives the translocation of the machine as it separates the two strands.
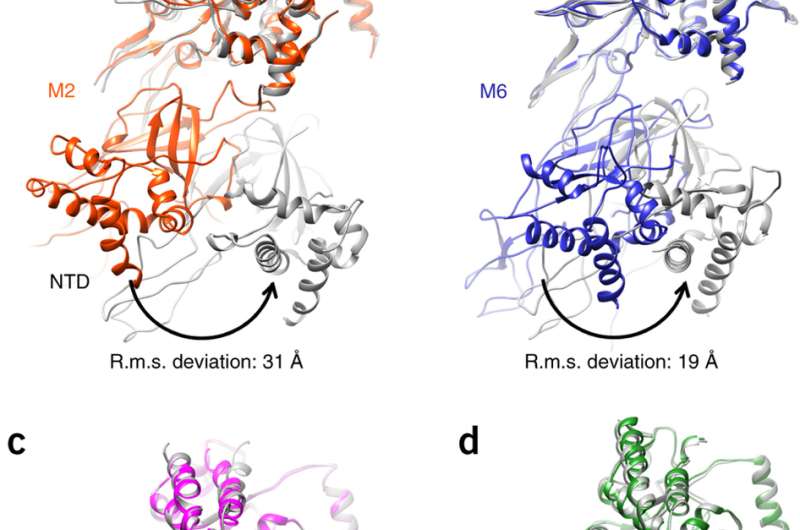
"Utilizing cryo electron microscopy, we were able to study closely the structure of the Mcm2-7 hexamer, which forms the core of the helicase," said HKUST professor Bik Tye. "We showed that the core is a left-handed coil or spring, not a closed ring as previously believed."
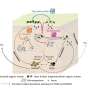
The minichromosome maintenance complex (MCM) hexameric complex (Mcm2-7) forms the core of the eukaryotic replicative helicase. During G1 phase, two Cdt1-Mcm2-7 heptamers are loaded onto each replication origin by the origin-recognition complex (ORC) and Cdc6 to form an inactive MCM double hexamer (DH), but the detailed loading mechanism remains unclear. Here we examine the structures of the yeast MCM hexamer and Cdt1-MCM heptamer from Saccharomyces cerevisiae. Both complexes form left-handed coil structures with a 10-15-Å gap between Mcm5 and Mcm2, and a central channel that is occluded by the C-terminal domain winged-helix motif of Mcm5. Cdt1 wraps around the N-terminal regions of Mcm2, Mcm6 and Mcm4 to stabilize the whole complex.
"The intrinsic coiled structures of the precursors provide valuable insights into the DH formation," said Dr. Yuanliang Zhai of HKUST, a co-author of the paper. "This suggests a spring-action model for the MCM during the initial origin melting and the subsequent DNA unwinding. "
Establishing the open coil as the ground state of the MCM hexamer has profound implications for the DNA-replication mechanism during both initiation and elongation.
First, upon activation of the DH, energy stored in the planar rings will be unleashed to provide the mechanical energy to uncouple the DH as each SH springs open to allow the escape of the melted single-stranded DNAs through the narrow Mcm2-Mcm5 gate to opposite sides. Second, the open-coil structure of the Mcm2-7 hexamer may determine the mode of action of the CMG helicase in its translocation along single-stranded DNA during DNA unwinding.
'This is all but a first step towards developing a thorough understanding of the action of Mcm2-7 complex in DNA unwinding," said professor Bik. "Ultimately, to determine the mechanism of ATP-hydrolysis-driven translocation of the catalytic MCM core, researchers will need high-resolution structures of the CMG helicase or the replisome in different functional states that will enable them to distinguish the identity of bound nucleotides at each ATPase center."
More information: Yuanliang Zhai et al, Open-ringed structure of the Cdt1–Mcm2–7 complex as a precursor of the MCM double hexamer, Nature Structural & Molecular Biology (2017). DOI: 10.1038/nsmb.3374
Journal information: Nature Structural & Molecular Biology
Provided by Hong Kong University of Science and Technology