Study details ringed structure of ORC in DNA replication
An international collaboration of life scientists, including experts at Van Andel Research Institute, has described in exquisite detail the critical first steps of DNA replication, which allows cells to divide and most advanced life, including human, to propagate.
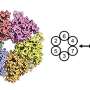
Results of the study are published in the journal Nature Structural and Molecular Biology and reveal that a ring-shaped protein called origin recognition complex (ORC) possesses a special alpha-helix, which slips into a groove on DNA and initiates a cascade of microscopic interactions that copy DNA.
"This is a story of one ring that lords over another ring," says Huilin Li, Ph.D., a professor in Van Andel Research Institute's Center for Epigenetics and a senior author of the paper. "Biologists have known for many years that both ORC and helicase are ring-shaped structures essential in the initiation and execution of DNA replication, but until now we never understood exactly how the ORC ring loads the helicase ring onto DNA."
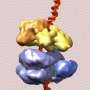
The work also reveals that ORC, with the help of Cdc6 and Cdt1, loads the helicase core onto DNA via paired interactions of the so-called winged helix domains. The resulting 14-protein structure completes the loading of the first helicase ring and is now prepared to load the next ring.
This process represents the inception of an immensely complex and elegant system that is constantly ongoing at tens of thousands of points on the DNA in many cells of the human body, and it all starts with ORCs.
"We hope that by mapping this process, others will eventually convert this knowledge into new treatments for DNA replication-related conditions, including many cancers and rare disorders," says Li.
At the outset, the six-protein ORCs assemble into a crescent, which envelops the DNA duplex. The ORCs then recruit a seventh protein, called Cdc6, to encircle DNA. Next, this ring threads the second ring, called minichromosome maintenance protein (Cdt1-bound Mcm2-7 hexamer), around DNA, which completes loading of the first Mcm2-7 hexamer.
"It's like threading a pearl onto a string; but unlike a short piece of string, the DNA strand is incredibly long and so the bead cannot be threaded on at one end," says Christian Speck, a professor at Imperial College of London's Institute of Clinical Sciences, leader of the DNA Replication group at MRC London Institute of Medical Sciences and a senior author of the paper. "Instead, it must somehow be opened up, slotted around the strand, and closed again."
The study was conducted on the DNA of Saccharomyces cerevisiae, better known as baker's yeast, because of its biological and genomic similarity to larger organisms, including mammals, at an average resolution of 3.9 Angströms (about 40 billionths of a meter), which is roughly the diameter of a single atom of sodium.
Magnification of this scale is currently possible only with cryoelectron microscopy (cryo-EM), a revolutionary technology VARI continues to invest in through its recently established Cryo-EM Core. Imaging for this study was conducted at Howard Hughes Medical Institute's Janelia Research Campus and at Scripps Research Institute.
More information: Zuanning Yuan et al. Structural basis of Mcm2–7 replicative helicase loading by ORC–Cdc6 and Cdt1, Nature Structural & Molecular Biology (2017). DOI: 10.1038/nsmb.3372
Journal information: Nature Structural and Molecular Biology , Nature Structural & Molecular Biology
Provided by Van Andel Research Institute