Coming together, falling apart, and starting over, battery style
Whether inside your laptop computer or storing energy outside wind farms, we need high-capacity, long-lasting, and safe batteries. In batteries, as in any electrochemical device, critical processes happen where the electrolyte and active material meet at the solid electrode. However, determining what happens at the meeting point has been difficult because in addition to active molecules, interfaces often contain numerous inactive components. Led by Laboratory Fellow Dr. Julia Laskin, scientists at Pacific Northwest National Laboratory have now found a way to carefully design technologically important interfaces by soft landing active molecules onto a small solid-state electrochemical cell. They packed the electrolyte into a solid membrane, deposited active ions on top, and characterized the cell using traditional electrochemical techniques. The device they built allows them to study key reactions in real time in controlled gaseous environments.
"To increase performance, we need to study what takes place inside batteries or fuel cells— understand processes at the interface in real time as the reactions are happening," said Dr. Venkateshkumar Prabhakaran, first author of the study.
The device provides a way to understand the basic breakdown reactions, material build-up, and other processes at the electrode surface during operation. Being able to gather this dynamic information is vital to building better batteries, fuel cells, and other energy devices. It also matters in improving the efficiency of industrial processes through electrocatalysis. "We are doing fundamental research on state-of-the-art technologically relevant interfaces," said Laskin.
At PNNL, scientists designed an electrochemical device to study the electrode-electrolyte interface in real time. The device uses a solid ionic-liquid membrane, in vacuum or other well-controlled environments, that has transport properties similar to a liquid electrolyte.
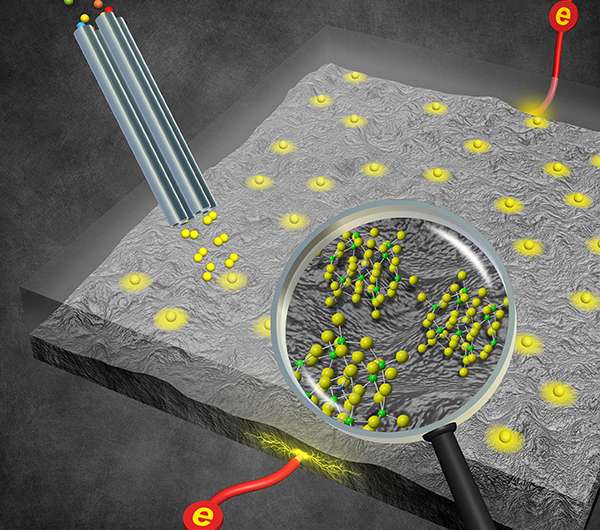
The solid membrane lets the team modify the electrolyte interface using ion soft-landing techniques. With soft landing, they place well-characterized active molecules at the interface. These molecules include catalytic metal clusters and redox-active "molecular battery" species capable of holding large numbers of electrons—potential candidates for boosting battery capacity.
In an exciting new twist, scientists can also add molecular fragments to the cell. They create the fragment ions by "smashing" precursor molecules in the gas phase. These gas-phase fragments may then be selected and added to the membrane. The result is a well-defined film that you can't typically make in solution. "This gives us access to a broad range of species that aren't stable under normal conditions and enables us to understand the contribution of individual building blocks to the overall activity of parent molecules," said Dr. Grant Johnson, a PNNL chemist and member of the team.
When the soft-landed clusters diffuse through the extremely thin membrane and reach the electrode surface of the newly designed device, the team has a detailed and precisely defined active species they can examine using several electrochemical and spectroscopic techniques. Once at the interface, the team can study how the active molecules change the transport of electrons, increasing capacity or depleting it, for example.
The researchers are using the device to study how soft-landed noble metal clusters modify carbon dioxide to upgrade this common pollutant to more valuable chemical feedstocks.
More information: Venkateshkumar Prabhakaran et al. In situ solid-state electrochemistry of mass-selected ions at well-defined electrode–electrolyte interfaces, Proceedings of the National Academy of Sciences (2016). DOI: 10.1073/pnas.1608730113
Journal information: Proceedings of the National Academy of Sciences
Provided by Pacific Northwest National Laboratory