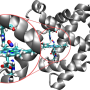
The discrepancy between the theoretical and experimental results of a system of biological interest is resolved
Research in which UPV/EHU's Department of Physical Chemistry collaborated has resolved the tautomeric equilibrium of a model system of great biological interest. The work was made possible using equipment designed to characterise sets of molecules with high accuracy. The research settles a controversy that existed between prior experiments and theoretical calculations that yielded contradictory and inconclusive results. This study has been selected for the front cover of the scientific journal Chemistry.
The study solves a problem that emerges between the different molecular structures in a tautomeric balance of great biological importance. It appears in DNA strands, for example. Tautomeric equilibrium is the name given to various structures that are differentiated only by the position of a functional group in which there is a chemical equilibrium between the structures and there is migration of an atom or group. The study targeted the 2-hydroxypyridine/2-pyridone model system, in which three structures coexist under the same molecular formula, differing in the positions of the atoms that comprise it. "As they have different structures, they will also perform different biological functions," explained Cocinero.
"The prior experiments yielded contradictory results indicating different structures as the predominant form and depending on how the experiment had been carried out. The theoretical calculations did not solve the problem either, because according to the methodology used, they once again yielded various responses, without being able to establish which one was the dominant form in this equilibrium that is hugely important in biological terms," explained Cocinero. Their research, however, has solved this situation.
More accurate characterisation
For their study, they took as the starting point the fact that the tautomeric equilibrium of this system largely depends on the medium in which it finds itself and whether it is in a solid state, in solution, or in the gas phase. In order to prevent interferences that could be exerted by the medium on this system, "the first thing was to characterise the structure of the molecules while keeping them isolated and taking a single molecule," explained Cocinero. For the characterisation, they used "microwave spectroscopy, the most accurate structural characterisation technique available, and which very few groups in the world use, as no commercial equipment exists," he added.
They carried out theoretical simulations taking the effects of different solutions into consideration. These calculations enabled them to correct the systematic error that was produced in the theoretical simulations using the values observed experimentally in the laboratory and they inserted corrections to predict other analogous systems.
Throughout the experimental work, they were particularly interested in determining the effect of adding a chlorine molecule to the system and gradually varying the position in which it was inserted into its structure. Cocinero says, "Chlorine appears in many chemical systems, and it is an easy way to modify the properties of the molecule by boosting one of the structures over the others and therefore modulating the function it is going to perform in nature. The inserting of atoms, groups or external elements is a widely-used methodology in the design of drugs to achieve the desired effect or response."
More information: Camilla Calabrese et al. Effects of Chlorination on the Tautomeric Equilibrium of 2-Hydroxypyridine: Experiment and Theory, Chemistry - A European Journal (2017). DOI: 10.1002/chem.201605977
Journal information: Chemistry – A European Journal
Provided by University of the Basque Country