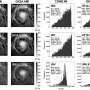
Researchers derive valuable chiral amino-alcohol structures from CO2
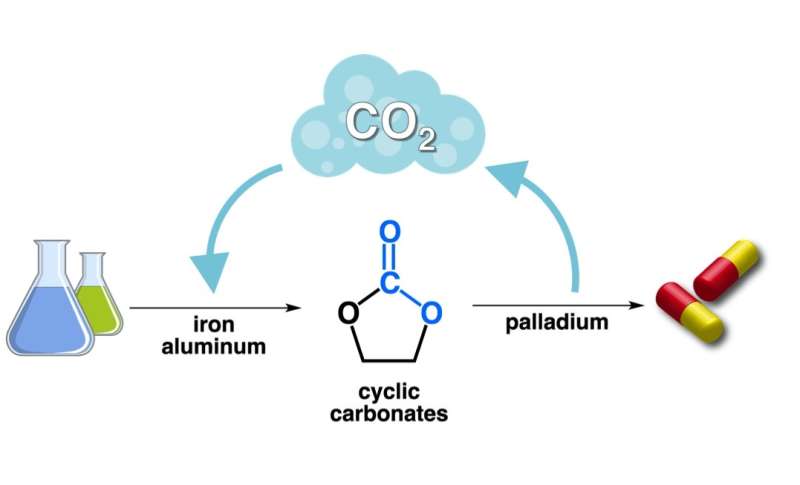
Researchers at the Institute of Chemical Research of Catalonia (ICIQ) in Tarragona have developed a method that transforms cyclic carbonates that can be easily obtained from CO2 into more valuable, chiral molecules chemists call vicinal amino-alcohols. Amino-alcohols are used in a myriad of drugs such as antimalarials, antivirals (like Tamiflu), analgesics and antiarrhythmics.
The group, led by Prof. Arjan Kleij, has developed new methodologies to convert small molecules like CO2 and other waste gases into useful chemicals. In previous work, they developed various catalytic routes to functional cyclic carbonates using carbon dioxide and easily accessible chemicals. Kleij and coworkers made these transformations possible with cheap and sustainable iron and aluminium catalysts.
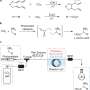
In a new paper recently published in the Journal of the American Chemical Society, ICIQ researchers show how to transform these carbonates into more valuable, highly challenging chiral molecules known as amino-alcohols. The process uses an efficient palladium catalyst, which is more abundant and less expensive than previously used rhodium alternatives. This reaction also releases CO2, which can be reused to make new carbonates, thereby closing the cycle.
"Chiral amino-alcohol structures are very common in pharmaceutical products. This new methodology allows us to obtain them selectively from simple, readily available starting materials," says Aijie Cai, who carried out the experiments. "Moreover, the method is quite user-friendly, it works without any additives or special precautions in just a few hours at 0°C," he adds.
Prof. Kleij, group leader at ICIQ, says, "Many years ago, we developed various efficient ways to prepare functional cyclic carbonates using CO2. Now, we are focusing on their post-synthetic conversion to create more challenging molecules with wider application potential. Chiral amino-alcohols are particularly valuable for the pharmaceutical industry, because they are precursors to important drugs such as antivirals and antimalarials."
CO2 is key to all this process; it is the philosopher's stone that transforms simple chemicals into valuable drugs. Kleij typically refers to it as "CO2 facilitated chemistry." Without carbon dioxide, none of the reaction steps would work. In the first step, researchers convert the gas into useful cyclic carbonates. During the second step, a palladium-catalysed CO2 elimination triggers the formation of the chiral products. The release of the gas allows the chemistry to move forward. CO2 could be recycled, making the overall process sustainable with minimal carbon emission.
More information: Aijie Cai et al, Palladium-Catalyzed Regio- and Enantioselective Synthesis of Allylic Amines Featuring Tetrasubstituted Tertiary Carbons, Journal of the American Chemical Society (2016). DOI: 10.1021/jacs.6b08841
Journal information: Journal of the American Chemical Society
Provided by Institute of Chemical Research of Catalonia (ICIQ)