Carbon dots dash toward 'green' recycling role
Graphene quantum dots may offer a simple way to recycle waste carbon dioxide into valuable fuel rather than release it into the atmosphere or bury it underground, according to Rice University scientists.
Nitrogen-doped graphene quantum dots (NGQDs) are an efficient electrocatalyst to make complex hydrocarbons from carbon dioxide, according to the research team led by Rice materials scientist Pulickel Ajayan. Using electrocatalysis, his lab has demonstrated the conversion of the greenhouse gas into small batches of ethylene and ethanol.
The research is detailed this week in Nature Communications.
Though they don't entirely understand the mechanism, the researchers found NGQDs worked nearly as efficiently as copper, which is also being tested as a catalyst to reduce carbon dioxide into liquid fuels and chemicals. And NGQDs keep their catalytic activity for a long time, they reported.
"It is surprising because people have tried all different kinds of catalysts. And there are only a few real choices such as copper," Ajayan said. "I think what we found is fundamentally interesting, because it provides an efficient pathway to screen new types of catalysts to convert carbon dioxide to higher-value products."
Those problems are hardly a secret. Atmospheric carbon dioxide rose above 400 parts per million earlier this year, the highest it's been in at least 800,000 years, as measured through ice-core analysis.

"If we can convert a sizable fraction of the carbon dioxide that is emitted, we could curb the rising levels of atmospheric carbon dioxide levels, which have been linked to climate change," said co-author Paul Kenis of the University of Illinois.
In lab tests, NGQDs proved able to reduce carbon dioxide by up to 90 percent and convert 45 percent into either ethylene or alcohol, comparable to copper electrocatalysts.
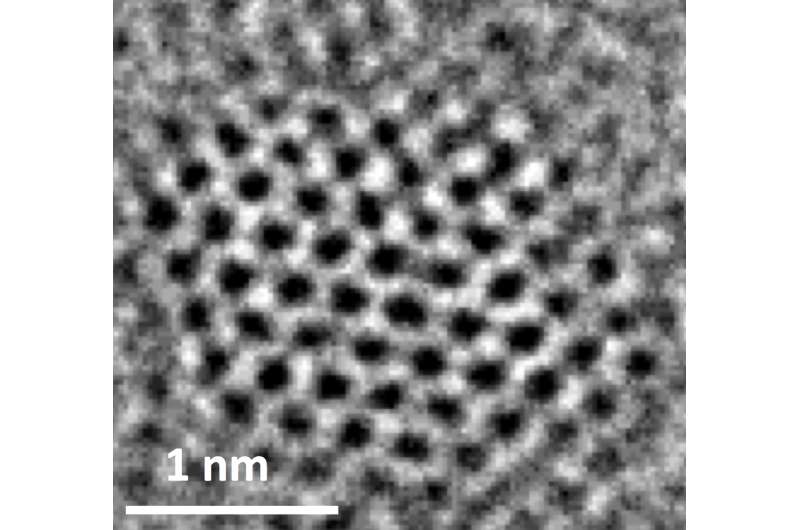
Graphene quantum dots are atom-thick sheets of carbon atoms that have been split into particles about a nanometer thick and just a few nanometers wide. The addition of nitrogen atoms to the dots enables varying chemical reactions when an electric current is applied and a feedstock like carbon dioxide is introduced.
"Carbon is typically not a catalyst," Ajayan said. "One of our questions is why this doping is so effective. When nitrogen is inserted into the hexagonal graphitic lattice, there are multiple positions it can take. Each of these positions, depending on where nitrogen sits, should have different catalytic activity. So it's been a puzzle, and though people have written a lot of papers in the last five to 10 years on doped and defective carbon being catalytic, the puzzle is not really solved."
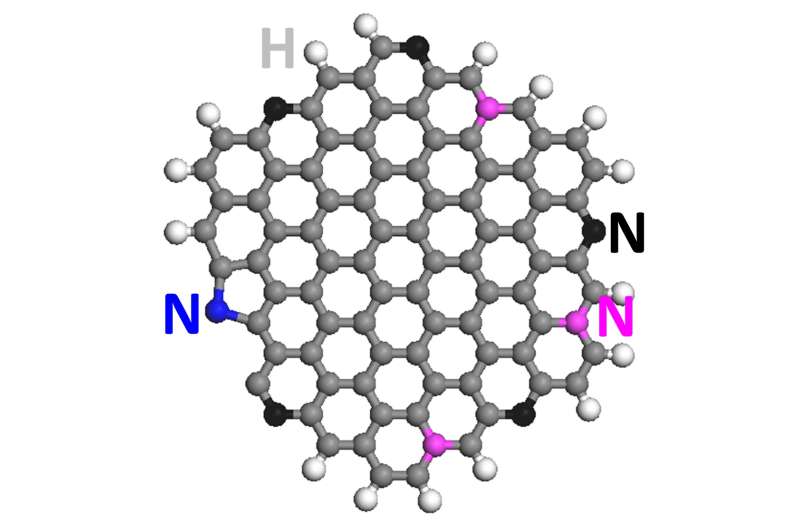
"Our findings suggest that the pyridinic nitrogen (a basic organic compound) sitting at the edge of graphene quantum dots leads the catalytic conversion of carbon dioxide to hydrocarbons," said Rice postdoctoral researcher Jingjie Wu, co-lead author of the paper. "The next task is further increasing nitrogen concentration to help increase the yield of hydrocarbons."
Ajayan noted that while electrocatalysis is effective at lab scales for now, industry relies on scalable thermal catalysis to produce fuels and chemicals. "For that reason, companies probably won't use it any time soon for large-scale production. But electrocatalysis can be easily done in the lab, and we showed it will be useful in the development of new catalysts."
More information: Jingjie Wu et al, A metal-free electrocatalyst for carbon dioxide reduction to multi-carbon hydrocarbons and oxygenates, Nature Communications (2016). DOI: 10.1038/ncomms13869
Journal information: Nature Communications
Provided by Rice University