Live cell imaging of asymmetric cell division in fertilized plant cells
A group of plant biologists at the Institute of Transformative Bio-Molecules (ITbM) of Nagoya University, the University of Tokyo, the Gregor Mendel Institute, and the University of Kentucky, have reported in the journal Proceedings of the National Academy of Sciences, on their discovery on how the plant's egg cells initially lose their skeletal pattern upon fertilization and are reorganized by two major cytoskeleton components in the cell, microtubules (MTs) and actin filaments (F-actin). Through live cell imaging, the group was able to visualize how fertilized egg cells in plants undergo asymmetric cell division, which is responsible for determining the plant's body axis.
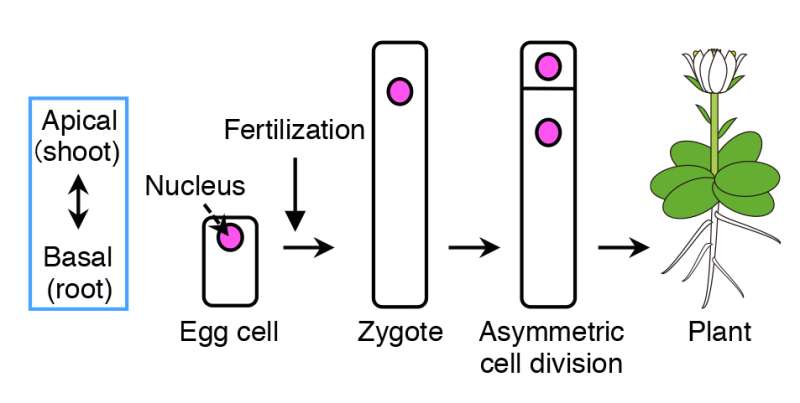
Flowering plants form various organs, such as the flower, leaves, root and stem, which develops along its body axis. As many plants take up a cylinder-like shape, the most important axis becomes the apical-basal (shoot-root) axis, i.e. the apical (top part) develops into shoots, containing flowers, stems and leaves, and the basal (bottom part) grows into roots. The fertilized egg cell (zygote), which is the origin for plants, establishes the plant's body axis from its first cell division.
Before cell division occurs, the contents within the zygote become unevenly distributed (polarized). This results in an unequal (asymmetric) cell division, generating a relatively small cell on top and a large cell at the bottom.
"Although polarization and asymmetric cell division of zygotes to form the body axis is a common phenomena found in algae, mosses, and flowering plants, the origin of cell polarity and how asymmetric cell division occurs have remained a mystery up to now," says Dr. Minako Ueda, a lecturer at ITbM, Nagoya University and a leader of this research. "The reason why this has been difficult was because there was not an efficient method to visualize the dynamics of cell division using the living zygote hiding deep inside the plants," she continues.
In 2015, Dr. Daisuke Kurihara's research group at Nagoya University reported a technique to visualize the growth of living embryos in a model plant, Arabidopsis thaliana (Arabidopsis). Ueda, Kurihara and their colleagues improved the resolution of this imaging technique to be able to observe the internal structure of the cell.
"The most difficult part of this research was to be able to identify the suitable markers to visualize the contents of the plant cell," explains Ueda. "With the help of Dr. Tomokazu Kawashima at the University of Kentucky and Professor Frederic Berger at the Mendel Institute, we tried different combinations of colored markers based on green fluorescent proteins (GFPs) to create a contrast between the different components within the cell. Yusuke Kimata, a graduate student in our group, conducted experiments to observe what was actually happening to the egg cell after fertilization."
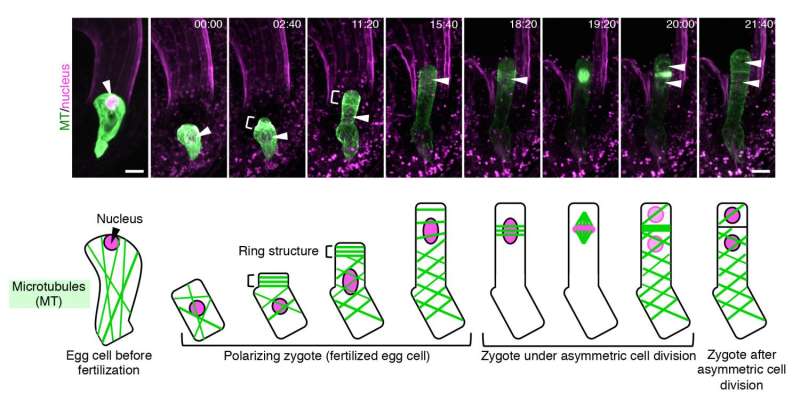
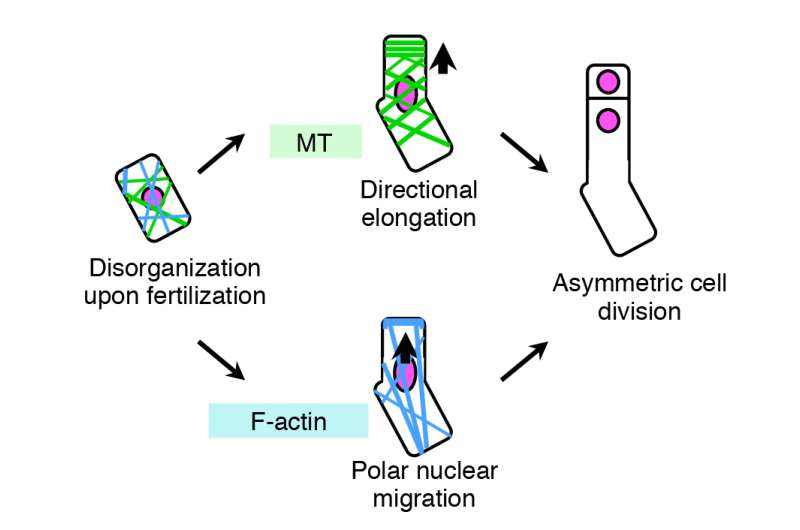
The group succeeded in visualizing for the first time, how the cytoskeleton of plant egg cells is disassembled after fertilization and then reorganized to create a polarity in the cell that eventually leads to asymmetric cell division. Plant cells contain two major cytoskeletons, i.e. microtubules (MTs) and actin filaments (F-actin), which help cells to maintain their shape, provide mechanistic support and enable the cells to divide and move. Ueda and Kimata used fluorescent markers of MTs and F-actin to see how they change before and after fertilization, and how the two fibers play a role in the polarization and asymmetric division of the zygote.
"From our live cell imaging experiments, we observed that MTs that were initially aligned along the top-bottom axis of the unfertilized egg cell, disintegrates upon fertilization, leading to shrinkage of the cell," describes Ueda. "After nearly 3 hours, a ring structure appeared at the top part of the zygote, from where a bulge appeared to elongate the cell. This ring structure was retained while the cell elongated. Finally, the MTs gathered around the nucleus after about 18 hours and distributed the chromosomes, eventually leading to cell division after about 22 hours," she continues. "We were really excited when we saw this movie, where the zygotes behave like a stretched Japanese rice cake, as this event was nothing like we have seen before."
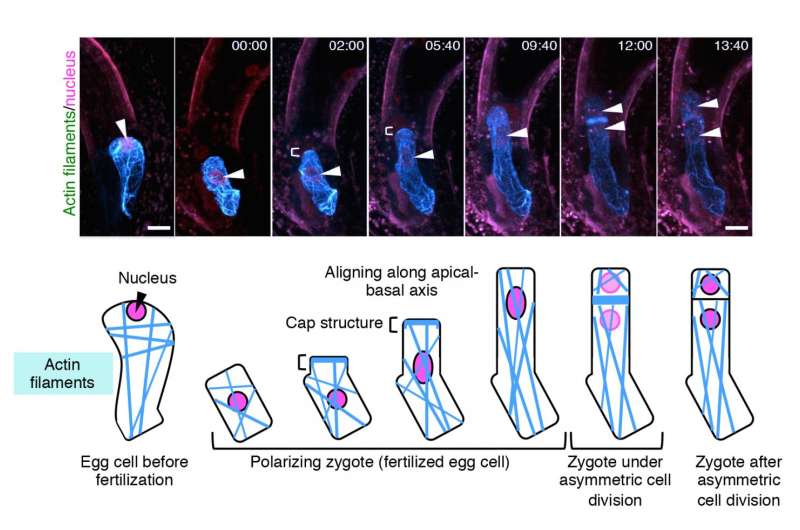
The group then studied the dynamics of F-actin by live imaging techniques. In a similar manner to MTs, the initial assembly of F-actin in an unfertilized egg cell was disrupted upon fertilization.
"What was different for F-actin, was that they align along the top-bottom axis after fertilization, and gather in a cap structure at the tip of the cell," describes Ueda. "We were able to observe that the initial assembly of both MTs and F-actin are disrupted upon fertilization of the egg cell, and the growing zygote gradually aligns these fibers in a different pattern from those in the egg cell. This is the first time to visualize the real time event of asymmetric cell division, and we were able to see other events such as cell elongation of the zygote and migration of the nucleus."
Not only did the group succeed in visualizing the real time events of zygote polarization and asymmetric cell division, they were able to quantify the dynamic patterns of the cytoskeleton (i.e. formation of the ring structure and longitudinal array of MTs and F-actin, respectively). Experts of image analysis, Dr. Takumi Higaki and Professor Seiichiro Hasezawa at the University of Tokyo, performed these detailed quantification experiments.
The group hypothesized that MTs and F-actin play different roles in the zygote due to their different alignment in the cell. In order to investigate their specific roles, they used inhibitors of each protein to see their effect on zygote polarization and asymmetric cell division.
"Through live imaging, we saw that inhibition of MTs hinders zygote elongation, resulting in formation of a round and swollen shape of the zygote head," describes Ueda. "On the other hand, when we inhibited F-actin, the nucleus was unable to move upwards and remained near the center of the zygote. As a result, cell division occurred at the position of the nucleus, leading to nearly symmetric cell division, where the generated cells were similar in size."
The group's results show that MTs are responsible for elongation of the zygote along the top-bottom axis, whereas F-actin plays a role in moving the nucleus towards the top part of the zygote, to make it ready for asymmetric cell division.
"We were able to show by live cell imaging that polarization of the cell occurs after fertilization of the egg cell, and both MTs and F-actin play a role in inducing asymmetric cell division to form the plant's body axis," says Ueda. "We hope to be able to find the exact origin of what causes polarization and the components that are being distributed in the cell by visualizing more components in the plant zygote. We envisage that this work will lead to discovering how flowering plants have evolved to form their current structure and shape."
More information: Yusuke Kimata et al, Cytoskeleton dynamics control the first asymmetric cell division inzygote, Proceedings of the National Academy of Sciences (2016). DOI: 10.1073/pnas.1613979113
Journal information: Proceedings of the National Academy of Sciences