The molecular mechanism that blocks membrane receptors involved in immunological response are identified
Nearly 70% of the drugs currently being developed target membrane receptors. Located outside the cell, these receptors play a decisive role in transmitting information to the inside of the cell. That is why in order to be able to advance in the development of more specific and more efficient drugs, the molecular mechanism that regulates the activity of these receptors needs to be deciphered. A piece of research in which the Ikerbasque researcher of the Biofisika Institute (UPV/EHU-CSIC) Xabier Contreras has participated has achieved a new advance when revealing how the receptors interact with the lipid nanodomains of the membrane. The work has been published in the prestigious journal Cell.
The study began by taking the medical history of 11 children, all of whom had a disorder due to mycobacteria infections, as the basis. All were discovered to have the same phenotype with the same mutation, which was located in the interferon-gamma (IFNGR) receptor, so the group began to explore what was causing this dysfunction.
The cell membrane can be likened to an ocean, a sea consisting mainly of lipids and proteins, in which there are islands made up of specific lipids such as cholesterol and sphingolipids. The membrane proteins are located on the islands and can only perform their function in these nanodomains.
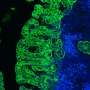
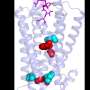
The IFNGR receptor is one of these membrane proteins and undertakes to activate genes involved in a huge variety of cell processes, including defence against pathogens and cancer. The team discovered that a simple mutation in the chain of 337 aminoacids that form it allows a sugar to be added. This sugar is recognised by a protein in the family of extracellular proteins known as galectins. When that protein is added to the receptor, the receptor gets taken out of its nanodomain and becomes caught up in the actin filaments that form the cell's cytoskeleton. Once outside its nanodomain, the receptor becomes blocked and can no longer transmit the signal.
"The research provides direct evidence on the fundamental role that certain lipid nanodomains play in the activation and regulation of cell signalling mediated by the IFNGR receptor. What is more, the results of this work stress the need to study the interaction between galectins and highly glycosylated membrane receptors and the link with various congenital diseases," pointed out Xabier Contreras. The study is also offering possible therapeutic targets for treating patients who are carriers of the IFNGR receptor mutation.
More information: Cédric M. Blouin et al. Glycosylation-Dependent IFN-γR Partitioning in Lipid and Actin Nanodomains Is Critical for JAK Activation, Cell (2016). DOI: 10.1016/j.cell.2016.07.003
Journal information: Cell
Provided by University of the Basque Country