Scientists detect thermal boundary that hinders ultracold experiments
Rice University scientists who analyze the properties of materials as small as a single molecule have encountered a challenge that appears at very low temperatures.
In trying to measure the plasmonic properties of gold nanowires, the Rice lab of condensed matter physicist Douglas Natelson determined that at room temperature, the wire heated up a bit when illuminated by a laser; but confoundingly, at ultracold temperatures and under the same light, its temperature rose by far more.
This is an issue for scientists like Natelson whose experiments require ultracold materials to stay that way. Laser heating, while it may seem minimal, presents a thermal barrier to simultaneous inelastic electron tunneling spectroscopy and surface-enhanced optical spectroscopy, which measure a material's electrical and optical properties.
Their report on the phenomenon appears in the American Chemical Society journal ACS Nano.
"Over the years we've made nice progress doing electronic and optical measurements simultaneously on nanoscale junctions that contain one or a few molecules," Natelson said. "We could learn a lot more if we could extend those measurements to quite low temperatures; the features in the electronic conduction would sharpen up a lot."
But such optical measurements require lasers, which combine with the properties of the metal electrodes to focus optical energy down to scales below the diffraction limit of light. "The laser for the optical measurements tends to heat the system," he said. "This isn't too bad at moderately low temperatures, but as we show in the paper, direct optical heating can get much more severe when the sample, without the light on, is cooled down to a few kelvins."
In plasmonic materials, lasers excite the oscillating quasi-particles that ripple like waves in a pool when excited. Plasmonic materials are used to sense biological conditions and molecular interactions; they also are used as photodetectors and have been employed in cancer therapies to heat and destroy tumors.
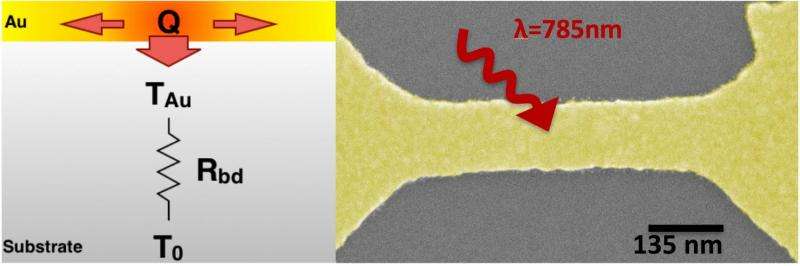
For their experiments, Natelson and his colleagues placed bowtie-shaped gold nanowires on silicon, silicon oxide, sapphire or quartz surfaces with a 1-nanometer adhesive layer of titanium between. They fabricated and tested 90 such devices. At their narrowest, the wires were less than 100 nanometers wide, and the geometry was tuned to be appropriate for plasmonic excitation with near-infrared light at 785 nanometers.
The researchers took measurements for various laser strengths and surface temperatures. For the nanowire on silicon or silicon oxide, they found that as they decreased the temperature of the silicon from 60 kelvins (-351 degrees Fahrenheit) to 5 kelvins (-450 F), it became less able to dissipate heat from the nanowire. With no change in the strength of the laser, the temperature of the wire increased to 100 kelvins (-279 F).
Replacing the silicon with sapphire provided some relief, with a threefold decrease in the laser-driven temperature increase, they reported. This was a startling result as the thermal conductivity of sapphire is a thousand times higher than that of silicon oxide, said Pavlo Zolotavin, a Rice postdoctoral researcher and lead author of the paper. A comprehensive numerical model of the structure revealed thermal boundary resistance as a major source of the detrimental temperature increase, especially for the crystalline substrates.
"The big issue is in getting vibrational heat out of the metal and into the insulating substrate," he said. "It turns out that this thermal boundary resistance gets much worse at low temperatures. The consequence is that the local temperature can get jacked up a lot with a somewhat complicated dependence, which we can actually model well, on the incident light intensity."
Solving the problem is important to Natelson and his team, as they specialize in measuring the electrical and magnetic properties of single molecules by placing them in gaps cut into bowtie nanowires. If heat expands the nanowires, the gaps close and the experiments are ruined. Heating can also "smear out" features in the data, he said.
"What this all means is that we need to be clever about how we try to do simultaneous electronic and optical measurements, and that we need to think hard about what the temperature distribution looks like and how the heat really flows in these systems," Natelson said.
More information: Pavlo Zolotavin et al. Plasmonic Heating in Au Nanowires at Low Temperatures: The Role of Thermal Boundary Resistance, ACS Nano (2016). DOI: 10.1021/acsnano.6b02911
Journal information: ACS Nano
Provided by Rice University



















