Lasers carve the path to tissue engineering
Future medicine is bound to include extensive tissue-engineering technologies such as organs-on-chips and organoids - miniature organs grown from stem cells. But all this is predicated on a simple yet challenging task: controlling cellular behavior in three dimensions. So far, most cell culture approaches are limited to two-dimensional environments (e.g. a Petri dish or a chip), but that neither matches real biology nor helps us sculpt tissues and organs. Two EPFL scientists have now developed a new method that uses lasers to carve out paths inside biocompatible gels to locally influence cell function and promote tissue formation. The work is published in Advanced Materials.
In the body, cells grow in 3D microspaces that are specific to each type of tissue - liver, kidney, lung, heart, brain etc. These microenvironments are important because they control the behavior of the cells, e.g. how they interact with other parts of the tissue to help it develop, function, and repair. In addition, the microenvironments themselves are very dynamic and adaptable, sending the cells various biochemical signals to adapt their behavior to physiological changes.
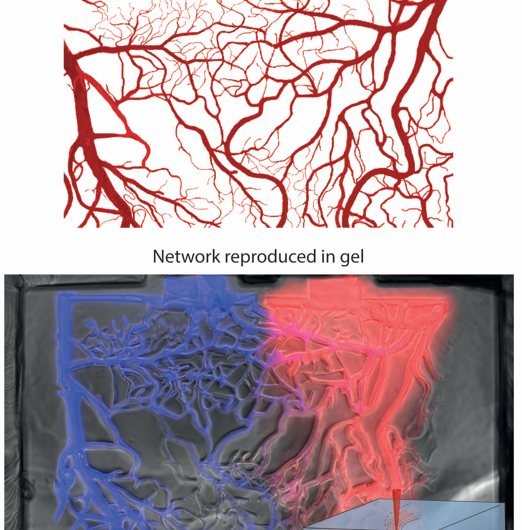
This means that any successful merging of biology and engineering must first be able to grow cells in custom-built yet biologically active 3D spaces. Working at EPFL's Institute of Bioengineering, Matthias Lütolf and his PhD student Nathalie Brandenberg have developed a method that uses a laser to cut three-dimensional pathways and networks for cells inside a hydrogel scaffold that matches their natural environment.
The method combines lasers with microfluidics - the science of controlling fluids in micrometer-sized spaces. The scientists used focalized short-pulsed lasers, which can generate enough power to create tiny tunnels in different gels already used in cell biology and tissue engineering. The laser can be applied before or even during 3D cell culture, meaning that the cells can be controlled in "real time" to match their natural growth.
Meanwhile, microfluidics have become the key to tissue engineering. The technology offers unprecedented control over the cells' microenvironment, as it can emulate the complex adaptability of biological microenvironments, allowing behavior-adjusting signals to be delivered to the cells in the form of drugs or other compounds.
As such, microfluidics are extensively used to build cell culture systems for growing cells. However, microfluidics have been largely limited to 2D cell culture applications, and are not easy to apply for long-term cell culture. Some efforts to use microfluidics in 3D cultures have proven successful, but they involve multiple labor-intensive steps that render them inefficient for standardized applications. But by combining microfluidics with the flexibility of laser carving (or "photoablation"), Brandenberg and Lütolf have brought ease, robustness and versatility to the approach.
"Our method addresses the limitations of previous approaches," says Lütolf. "It is fully compatible with 3D cell cultures, and can be applied with a wide range of materials, different geometries, and can introduce or change existing microfluidic networks during the course of an experiment to control cells in an unprecedented way."
More information: Brandenberg N, Lutolf MP. In Situ Patterning of Microfluidic Networks in 3D Cell-Laden Hydrogels. Advanced Materials 23 June 2016. DOI: 10.1002/adma.201601099
Journal information: Advanced Materials
Provided by Ecole Polytechnique Federale de Lausanne