Making organs transparent to improve nanomedicine
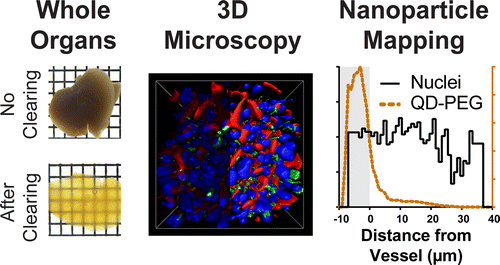
Treating a disease without causing side effects is one of the big promises of nanoparticle technology. But fulfilling it remains a challenge. One of the obstacles is that researchers have a hard time seeing where nanoparticles go once they're inside various parts of the body. But now one team has developed a way to help overcome this problem—by making tissues and organs clearer in the lab. Their study on mice appears in the journal ACS Nano.
Scientists are trying to design nanoparticles that deliver a therapeutic cargo directly to a disease site. This specific targeting could help avoid the nasty side effects that patients feel when a drug goes to heathy areas in the body. But barriers, such as blood vessel walls, can divert particles from reaching their intended destination. To get around such obstacles, scientists need a better understanding of how nanoparticles interact with structures inside the body. Current techniques, however, are limited. Warren C. W. Chan and colleagues wanted to develop a method to better track where nanoparticles go within tissues.
The researchers injected an acrylamide hydrogel into organs and tissues removed from mice. The gel linked all of the molecules together, except for the lipids, which are responsible for making tissues appear opaque. The lipids easily washed away, leaving the tissues clear but otherwise intact. Using this technique, the researchers could image nanoparticles at a depth of more than 1 millimeter, which is 25 times deeper than existing methods. In addition to helping scientists understand how nanoparticles interact with tumors and organs, the new approach could also contribute to tissue engineering, implant and biosensor applications, say the researchers.
More information: Shrey Sindhwani et al. Three-Dimensional Optical Mapping of Nanoparticle Distribution in Intact Tissues, ACS Nano (2016). DOI: 10.1021/acsnano.6b01879
Abstract
The role of tissue architecture in mediating nanoparticle transport, targeting, and biological effects is unknown due to the lack of tools for imaging nanomaterials in whole organs. Here, we developed a rapid optical mapping technique to image nanomaterials in intact organs ex vivo and in three-dimensions (3D). We engineered a high-throughput electrophoretic flow device to simultaneously transform up to 48 tissues into optically transparent structures, allowing subcellular imaging of nanomaterials more than 1 mm deep into tissues which is 25-fold greater than current techniques. A key finding is that nanomaterials can be retained in the processed tissue by chemical cross-linking of surface adsorbed serum proteins to the tissue matrix, which enables nanomaterials to be imaged with respect to cells, blood vessels, and other structures. We developed a computational algorithm to analyze and quantitatively map nanomaterial distribution. This method can be universally applied to visualize the distribution and interactions of materials in whole tissues and animals including such applications as the imaging of nanomaterials, tissue engineered constructs, and biosensors within their intact biological environment.
Journal information: ACS Nano
Provided by American Chemical Society