Engineers create custom tuning knobs to turn off any gene
Factory managers can improve productivity by telling workers to speed up, slow down, or stop doing tangential tasks while assembling widgets. Unfortunately for synthetic biologists attempting to produce pharmaceuticals, microbes don't respond to simple spoken directions like human personnel. Now, however, a new advance by University of Wisconsin-Madison engineers allows scientists fine-tune biological functions in their bacterial employees.
Synthetic biology has progressed by leaps and bounds since researchers first induced E. coli to make human insulin in the 1970s. Today, biological engineers coax microorganisms to perform numerous complex chemistries, such as breaking down plant material for biofuels. However, scientists still rely on a limited complement of components to control their synthetic circuits.
"We were frustrated because synthetic biology is littered with examples of artificial factors that can turn on and turn off gene expression under different conditions, but they only work for certain genes," says Brian Pfleger, the Walter J. and Cecile Hunt-Hougen Faculty Scholar and an associate professor of chemical and biological engineering at UW-Madison, the corresponding author on the paper.
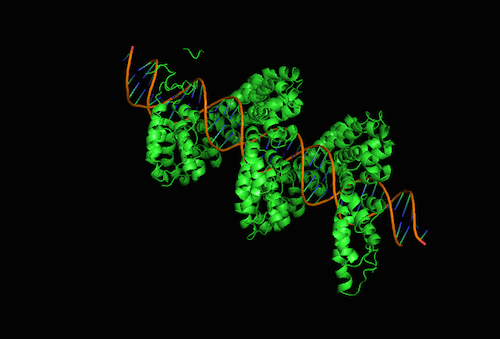
Pfleger's group developed a way to turn off almost any gene in E. coli. Their approach, published Feb. 8, 2016, in the advance online edition of the journal Nature Chemical Biology, borrowed a trick from nature to achieve human goals. The team modified proteins called TALEs to make tools that seek and silence diverse arrays of genes. TALE proteins come from bacteria that normally infect plants—the bugs typically inject TALEs into plant cells to make them more susceptible to infection.

"These pathogens piggyback on the molecular biology of higher organisms to manipulate them," says PhD student Mark Politz, the lead author on the paper. "It's kind of an ingenious little trick they're playing on their host."
Politz and Pfleger took advantage of the same sneaky strategy.
Natural TALEs attach to specific sections of plant genomes. The protein reads the DNA sequence and wraps itself around specific regions of the double helix like a boa constrictor. Once a TALE finds its target, it recruits the plant's own machinery to activate nearby genes. Previous research established which parts of the TALE recognize specific letters in the DNA alphabet, giving the Pfleger group a way to program these proteins to target any sequence they desired. Additionally they tweaked the TALEs so that the proteins repressed rather than promoted gene expression.
The engineered TALEs allowed them to temporarily turn off specific genes, without drastically altering the original organism.
"The great possibility for using TALEs is adding another layer of gene regulation that we can apply over top of what's already present in the natural bacteria," says Politz.
Scientists can easily erase genes by removing sections of DNA from some bacterial genomes. However, large deletions lack subtlety and often carry unforeseen consequences.
"Instead of going in and messing up all of the things nature has created in the cell, I just want to make less of a protein. And I want to do it in an inducible manner. I want to make less only when I want to make less," Politz says.
Counterintuitively, the most significant obstacle to creating the tool was finding a way to turn off the off-switch. Once TALEs wrap themselves around their target, they typically remain in position, exerting their effects indefinitely. In order to engineer a truly inducible system, the team introduced a deactivation mechanism.
The results established proof of principle in E. coli, one of the most commonly used organisms in modern biology. However, the researchers see applications for their approach across the tree of life.
"The tools that Mark's been developing are an example of what's taking place in biotechnology right now," Says Pfleger. "We're generalizing what we could do with organisms that have better inherent properties, like metabolisms that would be useful for biofuels."
More information: Matthew F Copeland et al. A transcription activator–like effector (TALE) induction system mediated by proteolysis, Nature Chemical Biology (2016). DOI: 10.1038/nchembio.2021
Journal information: Nature Chemical Biology
Provided by University of Wisconsin-Madison