Controllable protein gates deliver on-demand permeability in artificial nanovesicles
Researchers at the University of Basel have succeeded in building protein gates for artificial nano-vesicles that become transparent only under specific conditions. The gate responds to certain pH values, triggering a reaction and releasing active agents at the desired location. This is demonstrated in a study published in the journal Nano Letters.
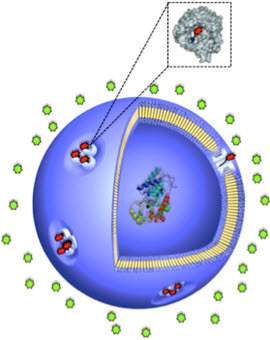
Tiny nanovesicles can protect active agents until they arrive in specific environments, such as at the target site in the body. In order to trigger a chemical reaction and release the contents at that loca-tion, the outer casing of the synthetically produced vesicles must become permeable at the correct point in time. Working under Prof. Cornelia Palivan, researchers from the Swiss Nanoscience Institute have now developed a membrane gate that opens on demand. This means that the enzymes inside a nanocapsule become active under exactly the right conditions and act on the diseased tissue directly.
Reacting to changes in pH
The gate is made up of the chemically modified membrane protein OmpF, which responds to certain pH values. At neutral pH in the human body, the membrane is impermeable – but if it encounters a region with acidic pH, the protein gate opens and substances from the surrounding area can enter the nanocapsule. In the resulting enzymatic reaction, the capsule's contents act on the incoming substrate and the product of this reaction is released. This method could be applied, for example, to inflamed or cancerous tissue, which often exhibits a slightly acidic pH value.
Until now, permeability in nanovesicles has been achieved using natural proteins that operate as pores in the protective membrane, allowing both the substrate to enter and the product of the enzymatic reaction to escape. However, fields such as medicine or controlled catalysis call for more precise distribution in order to achieve the greatest possible efficiency of the active agent. In collaboration with Prof. Wolfgang Meier's team, the chemists working under Prof. Palivan were able for the first time to integrate a modified membrane protein into an artificially produced nanocapsule, which opened only if it encountered corresponding pH values.
More information: Tomaz Einfalt et al. Stimuli-triggered activity of nanoreactors by biomimetic engineering polymer membranes, Nano Letters (2015). DOI: 10.1021/acs.nanolett.5b03386
Journal information: Nano Letters
Provided by University of Basel