Tweaking proteins with 'Tub-tag'

LMU researchers, together with colleagues based in Berlin, have developed a rapid and efficient technique for targeted chemoenzymatic functionalization of proteins. The new method has a wide range of potential therapeutic applications.
Selective intermolecular recognition is at the heart of all biological processes. Thus proteins that bind specifically to complementary chemical structures are also indispensable for many biochemical and biotechnological applications. Targeted modification of such proteins therefore plays a significant role in medical diagnostics and therapies. Now researchers led by Professor Heinrich Leonhardt at LMU's Biocenter and Professor Christian Hackenberger of the Leibniz Institute for Molecular Pharmacology in Berlin have developed a new strategy that permits specific chemical modification of virtually any protein more rapidly and more efficiently than was hitherto possible. Their results appear in the new edition of the journal Angewandte Chemie.
Many of the methods routinely used in the biosciences are based on the specific modification of proteins, in particular antibodies, to endow them with novel properties for specific purposes. For example, chemotherapeutic agents used in the treatment of cancer are often chemically linked to antibodies that recognize antigens found only on the surface of the target tumor. In this way, the cytoxic drug can be delivered directly to the cells it is intended to eradicate. Ideally, the methods used to introduce such modifications should be as specific, efficient and versatile as possible. Unfortunately, the techniques currently in use fulfill these criteria only in part. "Thanks to the combination of biotechnological and chemical expertise available for this cooperative project, we have succeeded in developing what we call the 'Tub-tag' technology, which is characterized by extremely high efficiency and tremendous chemical flexibility and is simple to perform," says Hackenberger.
Guide sequence integrated
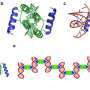
The new method is the first to make use of the enzyme tubulin-tyrosine ligase (TTL) and its target sequence. TTL binds to a short amino-acid sequence found in its natural target - the cytoskeletal protein tubulin - and adds the amino-acid tyrosine to its C-terminal end. The researchers therefore refer to this guide sequence as 'the Tub-tag'. "Our idea was to integrate this Tub-tag sequence into other proteins, thus turning them into targets for the enzyme TTL. We have demonstrated the feasibility of this approach by introducing the Tub-tag into various so-called nanobodies, which are downsized and stable derivatives of antibodies that we have used using with great success in our laboratory for many years," Leonhardt explains.
Adaptor for attachment of reactive agents
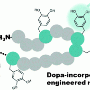
Since the engineered nanobodies are now recognized as targets by TTL, the enzyme can also be used to attach synthetic derivatives of tyrosine to them. "We can then exploit these 'unnatural' tyrosine derivatives as chemical adaptors. In a subsequent step, with the aid of various well established chemical methods, we can then add virtually any molecule with the required properties to the appropriate adaptors," says Dominik Schumacher, joint first author of the study.
The technique can be employed in a wide variety of contexts. One obvious and highly promising application is in the production of so-called antibody-drug conjugates (ADCs) for use in tumor therapy. As mentioned above, ADCs enable cytotoxic agents to be transported directly to the tumor tissue, thus minimizing deleterious side-effects. "But the relative lack of efficient ways to attach chemotherapeutic drugs to antibodies currently represents a major technological bottleneck," says Dr. Jonas Helma, also joint first author of the new publication. "We now offer a novel and superior technology for this task."
More information: "Versatile and Efficient Site-Specific Protein Functionalization by Tubulin Tyrosine Ligase." Angewandte Chemie 25 SEP 2015 DOI: 10.1002/anie.201505456
Journal information: Angewandte Chemie
Provided by Ludwig Maximilian University of Munich