September 28, 2015 feature
Alloy engineering addresses long-standing problem of semiconductor defects
(Phys.org)—The performance of all of today's electronic devices depends on the quality of the semiconductor materials they're made of. Two of the most important factors that affect a semiconductor's properties are its band gap and its defects, both of which can be tuned to control its conductivity. While several methods exist to tune band gaps, there is still a lack of effective methods for controlling defects, which can have adverse effects on the semiconductor's overall properties.
In a new paper published in Physical Review Letters, physicists Bing Huang, et al., have demonstrated that alloy engineering—here, the mixing of two semiconductors—can reduce the levels of deep-level defects and their adverse effects without significantly changing the semiconductor's basic electronic structure. In previous strategies to control defects, the electronic structure is inadvertently changed, which leads to additional problems.
"So far, there is no successful concept that we can use to tune the defect properties of semiconductors without changing the electronic properties of the host materials," Huang, from the Beijing Computational Science Research Center, the University of Utah, and Oak Ridge National Laboratory, told Phys.org. "Our concept is the first demonstration for this long-standing question in semiconducting physics. We show that the mixing of two similar semiconductors can have exceptional benefits on the defect properties of the host materials."
As the scientists explain, this advantage is surprising, and arises from the fact that some physical properties (the electronic structure) depend on the global concentration of the alloy, whereas other physical properties (defects) depend only on the local concentration of the alloy.
To demonstrate the new method, the researchers fabricated alloys of a newer type of semiconductor called transition metal dichalcogenides (TMDs) by mixing two TMDs, MoSe2 and WSe2, in different concentrations. TMDs have gained attention in recent years because they consist of atomically thin monolayers, like graphene, but unlike graphene they have a non-zero band gap and so are good semiconductors.
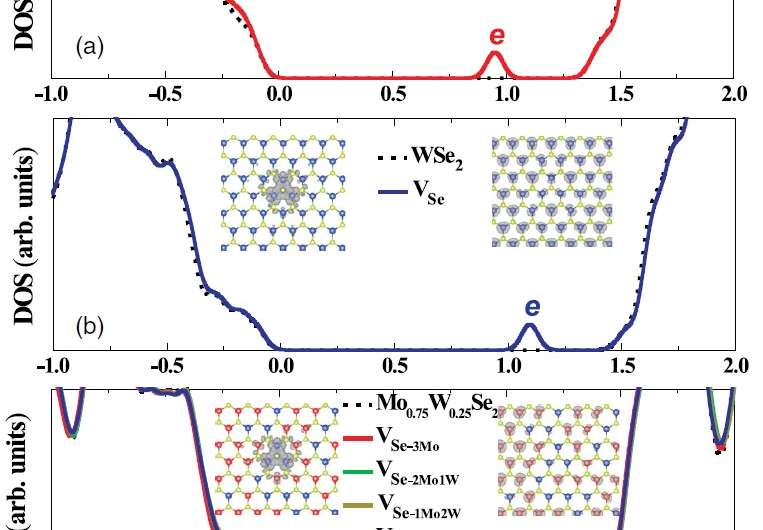
The most abundant and harmful defect in TMDs are anion vacancies, which occur in both MoSe2 and WSe2 as missing Se (selenium) ions. These vacancies can scatter or trap the charge carriers, such as the electrons and holes, which decreases conductivity. Se vacancies also form more easily around W (tungsten) atoms than Mo (molybdenum) atoms, and so are mainly determined by their local environment, not the total W concentration.
What the researchers showed here sounds somewhat counterintuitive at first: while the defect levels of the Se vacancies are very deep in both MoSe2 and WSe2, they become much shallower, and therefore less harmful, when these two TMDs are mixed together at low W concentrations. One example is the alloy Mo0.75W0.25Se2, where the decimals indicate the percentage composition of the whole quasirandom alloy structure, in which each Se atom is surrounded by a total of three Mo and/or W atoms.
Huang explained that the alloying method works so well due to the different effects of the global W concentration and the local W positions.
"As we increase the W concentration in the Mo1-xWxSe2 alloy, the global conduction band position of the alloy will be increased continuously, as it only depends on the concentration of W," he explained. "However, the defect level is determined only by the local environment around the defect, e.g., in our case, the defect level of Se vacancies is solely determined by how many W atoms are around it, and this has nothing to do with the W concentration."
On a physical level, the reason why this alloy has fewer defects than either of its component parts is due to the fact that a combination of Mo and W atoms around a Se vacancy breaks the symmetry of the chemical structure, which in turn splits the defect state into two shallower states. At the same time, the alloy of MoSe2 and WSe2 (which have very similar electronic structures and band gaps) maintains these electronic properties. The overall result is an improvement in the carrier density by five orders of magnitude, which generally leads to a significant improvement in device performance.
The researchers expect that this method of alloy engineering to suppress defects can be extended to other TMDs with similar electronic structures, and even to semiconductors with dissimilar electronic structures as long as the average electronic properties remain useful.
"We are using this new concept to investigate the possibility of tuning the defect level positions in other critical semiconductors, like ZnO and CdTe," Huang said. "In these materials, the conventional doping methods are invalid. For example, we cannot make ZnO p-type-doped now, but this is very critical for the use of ZnO in the semiconductor industry."
More information: Bing Huang, et al. "Alloy Engineering of Defect Properties in Semiconductors: Suppression of Deep Levels in Transition-Metal Dichalcogenides." Physical Review Letters. DOI: 10.1103/PhysRevLett.115.126806
Journal information: Physical Review Letters
© 2015 Phys.org