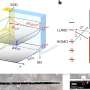
Reaction to convert solar energy to fuel is 50 times faster with a simple change in the solvent
Few people would buy a computer that only worked when the sun shines. For solar energy, that's the problem. Energy produced at solar stations on sunny days must be stored for later use. A simple fuel, such as hydrogen, could store the energy. Making hydrogen economically demands a quick, efficient reaction. Creating that reaction demands a catalyst, which pushes the reaction along, in the right environment. Scientists at the Center for Molecular Electrocatalysis (CME) found that a proton and water-packed environment lets the catalyst work 50 times faster than the previous record holder—without added energy.
"It is really remarkable how much the media are controlling the rates," said Dr. Molly O'Hagan, who led the study at CME. The center is led by Pacific Northwest National Laboratory.
No bank of batteries sits under solar stations or wind turbines. Coupling renewable energy with fuel production could solve the storage issue. The study provides a vital clue to that coupling: placing the catalyst in a proton-packed liquid with plenty of water produces hydrogen fuel quickly and efficiently. Doused in this designer liquid, the catalyst pumps out 30,000,000 hydrogen molecules a second.
"Our center is focused on designing catalysts that control proton mobility," said Dr. R. Morris Bullock, CME Director. "Here, we are using the medium, an ionic liquid with water, to control the proton movement."
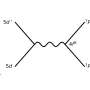
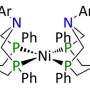
At the CME, an Energy Frontier Research Center funded by the U.S. Department of Energy Office of Science's Basic Energy Sciences, researchers examine how to control proton movement in catalysis. In this experiment-based study, they began with a nickel-based P2N2 hydrogen production catalyst. The team observed that the catalyst worked quickly, or had a large turnover rate, when water was added to an ionic liquid.
"This solvent, or ionic liquid, is like dissolving the catalyst in pure substrate, but the catalyst won't work in this liquid without adding water which is necessary to move the substrate very quickly to the catalyst," said O'Hagan. "Putting the catalyst in this mixture increased speed dramatically without increasing the energy cost."
The team is combining proton control inside the catalyst with proton control in the environment. The goal? Provide the design specifications to create efficient, super-fast catalysts that turn solar stations into fuel stations.
More information: "Electrocatalytic H2 Production with a Turnover Frequency >107 s-1: The Medium Provides an Increase in Rate but Not Overpotential." Energy & Environmental Science 7:4013. DOI: 10.1039/c4ee01899k
Journal information: Energy & Environmental Science
Provided by Pacific Northwest National Laboratory