Hydrogels block harmful oxygen from sensitive catalysts
An international research team has found a way of protecting sensitive catalysts from oxygen-caused damage. In the future, this could facilitate the creation of hydrogen fuel cells with molecular catalysts or with biomolecules such as the hydrogenase enzyme. To date, this could only be accomplished using the rare and expensive precious metal platinum. Together with their French colleagues, researchers from Bochum and Mülheim describe the way in which a hydrogel can serve as a "protective shield" for biomolecules by two articles written in the journals Angewandte Chemie and the Journal of the American Chemical Society.
Requirements on catalysts are difficult to reconcile
In order to be suitable for industrial applications, catalysts have to be efficient, stable and affordable; in addition, they have to be tailor-cut for one specific chemical reaction. "Uniting all of these requirements in one molecule is a considerable challenge," says Dr Nicolas Plumeré from the Chemistry Department at the Ruhr-Universität Bochum. However, a novel hydrogel in which catalysts are embedded could greatly simplify the development of fuel cell catalysts in the future. To explore this possibility, the researchers from Bochum began a collaborative project with colleagues from the Max Planck Institute for Chemical Energy Conversion in Mülheim and from Aix Marseille University and the Centre National de la Recherche Scientifique (CNRS) in France.
Hydrogel acting as solvent and as protective environment
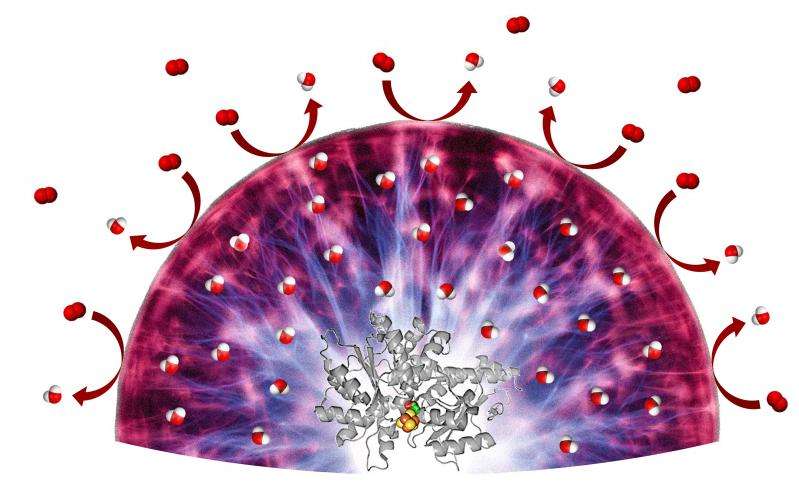
For their experiments, the German team utilised the hydrogenase enzyme from the green alga Chlamydomonas rheinhardtii; it splits hydrogen into protons and electrons. Typically, even trace amounts of oxygen cause irreversible damage to this biomolecule. However, the researchers incorporated it in a hydrogel which assumes two functions: it acts as a solvent, ensuring that all reaction partners reach the enzyme quickly and easily. At the same time, it provides a protective environment in which the oxygen cannot penetrate through to the enzyme, even if it is present at relatively high concentrations. The trick: the hydrogenase activity leads to the creation of electrons; they wander through the hydrogel and are transmitted to the oxygen, thus converting it into a harmless form, namely water.
Catalyst design could become considerably easier in the future
Using simulations and experiments, the German-French team demonstrated another important property of hydrogels. The activity of many catalysts decreases over time due to exposure to deactivating molecules. Some can be rendered functional again through special reactivation processes. Notably, however, the hydrogel protects even those catalysts for which a reactivation process does not exist. "In future, we will thus no longer have to pay attention to the robustness or suitable reactivation processes when developing catalysts for technical applications," explains Olaf Rüdiger, Chemist at the Max Planck Institute for Chemical Energy Conversion. "We can focus solely on maximising the catalyst's activity. This will simplify the development process to a considerable degree and open up new possibilities for the manufacture of fuel cells."
More information: "The mechanism of protection of catalysts supported in redox hydrogel films," Journal of the American Chemical Society, DOI: 10.1021/jacs.5b01194
Journal information: Angewandte Chemie , Journal of the American Chemical Society
Provided by Ruhr-Universitaet-Bochum