Researchers find less expensive way to convert carbon dioxide
With an abundance of carbon dioxide being produced worldwide, scientists and engineers are looking for inexpensive ways to turn it into something useful, such as hydrocarbon fuels.
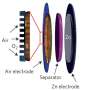
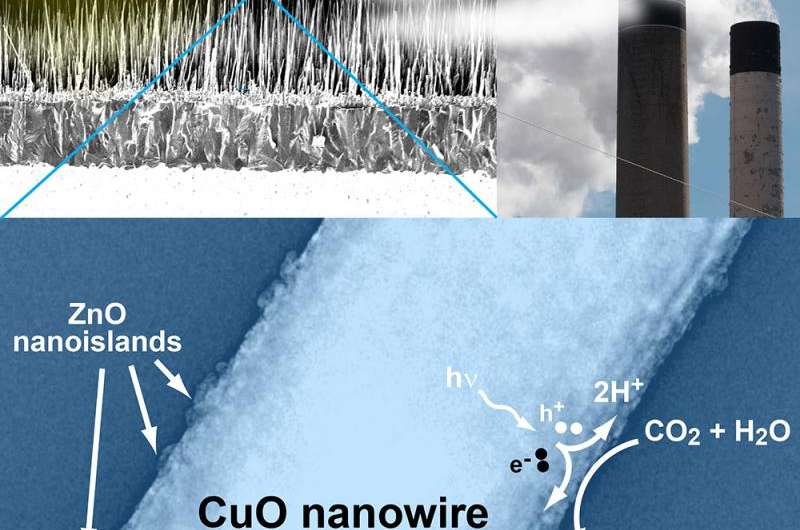
A collaboration of researchers at Washington University in St. Louis and Korea University used copper oxide nanowires as a catalyst to convert carbon dioxide into carbon monoxide, which can then be used as a feeder material to create plastics and higher-carbon polymers. The reduction of carbon dioxide is a very energy-intensive process, so the researchers have developed a method to tap solar energy to allow the conversion.
Results of the research were published Feb. 27 in ACS Applied Materials & Interfaces.
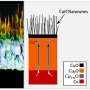
Parag Banerjee, PhD, assistant professor of materials science in the School of Engineering & Applied Science, works with nanowires created from copper oxide coated with zinc oxide. Banerjee and his lab worked with Pratim Biswas, PhD, the Stanley & Lucy Lopata Professor and chair of the Department of Energy, Environmental & Chemical Engineering.
In this collaboration, the two labs compared two different methods of using nanowires to convert carbon dioxide to another material. Previously, Biswas' lab developed various nanostructured materials for the conversion of carbon dioxide and showed that they could create nano- and meso-structures with titanium dioxide coated with platinum, animated-graphene-encapsulated photocatalysts and others.
"Platinum is expensive, therefore the process would be difficult to scale up," said Banerjee, who runs the Laboratory for Emerging and Applied Nanomaterials (LEAN). "So one has to look into earth-abundant and cheap materials to address conversion of CO2 at the industrial and even global scale."
Banerjee said there are plusses and minuses to both processes.
"The main advantage of the platinum process is that you can go from carbon dioxide to methane in one step," he said. "That takes eight electrons, which is a lot of electrons. The zinc oxide process takes only two electrons, but converts carbon dioxide into carbon monoxide and doesn't require platinum, so there are trade-offs."
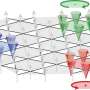
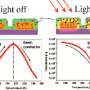
To create the catalysts, Banerjee and his lab used copper oxide nanowires, which absorb the light and create electrons and holes. A few atomic layers of zinc oxide on top of the wires allow these electrons to stay on the surface for a long time.
"If you have free electrons, you can initiate chemical reactions," he said. "That's what this catalyst does—it enables electrons to react with gas molecules, which stick to the catalyst surface. Without the zinc oxide added to the copper oxide, this wouldn't work. And using zinc oxide alone, it wouldn't work either, because you need the right material to absorb light, and that's what the copper oxide does. Everything has its own role to play in this catalyst."
Banerjee credits members of his and Biswas' lab, as well as the Photosynthetic Antenna Research Center (PARC) at Washington University, for the successful results.
"Wei-Ning Wang, Fei Wu and Yoon Myung formed an excellent team," Banerjee said. "They showed immense coordination and drive to bring this work to fruition. Research was coordinated across three labs at WashU and one lab in Korea. PARC's support on this project was critical, as well, and having such a facility on campus helps us all do world-class research."
More information: "Surface Engineered CuO Nanowires with ZnO Islands for CO2 Photoreduction." ACS Applied Materials & Interfaces. Feb. 27, 2015. DOI: 10.1021/am508590j
Journal information: ACS Applied Materials and Interfaces
Provided by Washington University in St. Louis