The taming of the shrew: Scientists decipher the spectrum of CH5+ for the first time
For the first time ever, a team of scientist from the University of Cologne headed by Professor Stephan Schlemmer succeeded in understanding the spectrum of the highly fluxional molecule CH5+. This insight, gained in collaboration with a Japanese colleague, was made possible by the extreme cooling of this enigmatic molecule and a highly accurate measurement of its vibrational transitions. The results will be presented on March 20, 2015 in Science magazine.
CH5+, formed by adding a proton (H+) to the well-known methane (CH4) molecule, is the prototype of fluxional molecules. In contrast to common molecules, which are depicted as a rigid structure consisting of balls (atoms) and sticks (chemical bonds), the five hydrogen nuclei in CH5+ can move quite freely around the carbon nucleus. It is thus constantly in motion, even at an extremely low temperature. Bonds are broken and reformed all the time, and therefore the simple model of balls and sticks does not apply. There has thus been a long debate whether CH5+ has a structure at all.
This extraordinary fluxional behavior is reflected in the spectra of CH5+. Usually such spectra are recorded in the lab to characterize and identify molecules. With the help of suitable theoretical models, the vibrational spectra can yield information about bond strengths and molecular structure. For CH5+, however, the hitherto known spectra have been so chaotic that not a single of the many hundred vibrational transitions could be understood or assigned. This has been considered one of the last mysteries of molecular physics.
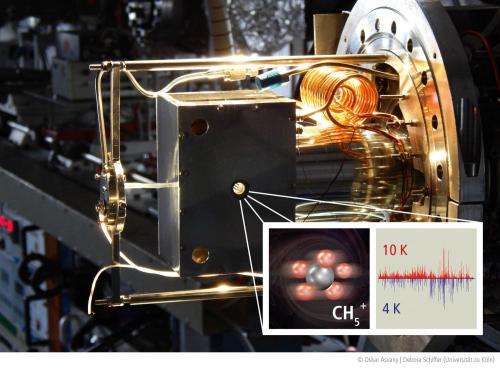
By developing and applying new ion trap experiments, physicists from the University of Cologne have now succeeded in storing a pure sample of CH5+ ions and cooling it down to a temperature close to absolute zero. With the help of a so-called frequency comb, the vibrational transitions could be measured with high accuracy, leading to a reconstruction of the lowest energy levels. This very technical approach was necessary due to the complete lack of theoretical models for this exceptional molecule. The results are thus based only on the experimental data and the fundamental principle of quantum mechanics, according to which the observed vibrational transitions are based on a scheme of discrete energy levels.
Surprisingly, the results are in accordance with the simple notion that the five hydrogen nuclei can move quite freely around the central carbon nucleus, with their distance to it being more or less fixed. Whether this simple picture is valid will have to be tested in further investigations. In any case, the highly accurate data will challenge future theoretical models to interpret the discovered energy levels. The entire class of fluxional molecules will profit from these developments.
More information: Taming CH5+, the "enfant terrible" of chemical structures, Science, www.sciencemag.org/lookup/doi/ … 1126/science.aaa6935
Journal information: Science
Provided by University of Cologne