TLR9: Two rings to bind them?
University of Tokyo researchers have elucidated how Toll-like receptor 9 (TLR9) binds to pathogen DNA, activating the innate immune system. This discovery is vital for the design of new antiviral, antibacterial, allergy and other drugs targeting TLR9.
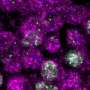
Invading pathogens such as bacteria or viruses leave traces in the form of DNA fragments, proteins and other biomolecules. TLR9 is a membrane-bound protein that detects these traces by recognizing a DNA sequence called Cytosine-phosphate-Guanine dinucleotide (CpG), a motif that is specific to bacteria and viruses. This brings about the release of interferon and induces inflammation, but until recently, the structure of TLR9 and how it functions remained unknown.
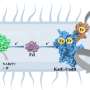
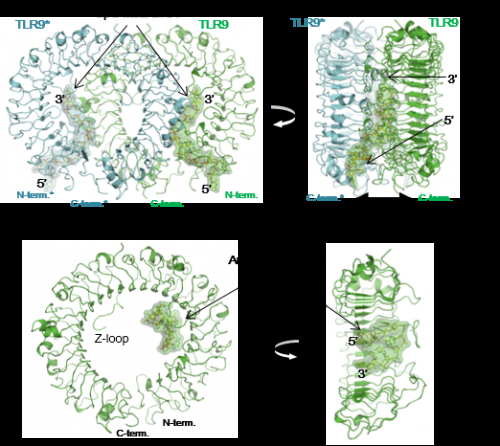
Professor Toshiyuki Shimizu's research group at the Graduate School of Pharmaceutical Sciences, working with researchers at the University of Tokyo's Institute of Medical Science and Osaka University's Graduate School of Engineering, determined the structure of the ring-shaped TLR9 structure in three important forms: as a free protein, bound to an inhibitor DNA, and bound to an agonist (a molecule that activates TLR9 as a pathogen) DNA. In the first two cases, TLR9 exists as a single ring, but when bound to an agonist, such as a segment of DNA containing the CpG motif, two of its rings are bound together to form a dimer sharing two DNA molecules. The researchers used beamlines at Photon Factory and SPring-8 to obtain crystallographic data in making this achievement.
"TLR9 is a promising drug target for treating viral infections, cancer, autoimmune diseases, and so on, so researchers have been trying to elucidate structurally how TLR9 recognizes pathogenic DNA ever since it was discovered more than a decade ago. This work represents a big step forward for drug development targeting TLR9, and also for our understanding of nucleic acid sensing by TLR9," says Professor Shimizu. "TLRs have received significant attention due to their critical roles in the innate immune system, and our group has been focusing on the structural study of TLRs for many years. We believe that a precise understanding of TLR function should come from its visualization by structural analyses. Actually, we were quite surprised at the result of this study: the two DNA molecules, agonist and inhibitor, bound to completely different sites on TLR9, and the DNA molecules themselves had completely different structures, both of which we could never have predicted."
More information: Umeharu Ohto, Takuma Shibata, Hiromi Tanji, Hanako Ishida, Elena Krayukhina, Susumu Uchiyama, Kensuke Miyake, and Toshiyuki Shimizu, "Structural basis of CpG and inhibitory DNA recognition by Toll-like receptor 9", Nature, DOI: 10.1038/nature14138
Journal information: Nature
Provided by University of Tokyo