A library of sequences that modify relative gene expression enables tighter control over protein production
Reprogramming a cell to express a foreign gene is relatively straightforward. However, reprogramming a cell to express multiple foreign genes is far more complex, especially if trying to exert control over the interplay between these genes. Now, researchers led by Yuansheng Yang of the A*STAR Bioprocessing Technology Institute, Singapore, have developed a strategy to finely control the relative activity of multiple genes in parallel.
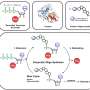
Some of the complexity of reprogramming lies in balancing proportions: many proteins consist of multiple component subunits, and for the complex to assemble properly, each subunit must be manufactured in specific proportions. Yang's strategy was to manipulate an RNA sequence known as an internal ribosome entry site (IRES). Most messenger RNA (mRNA) molecules represent the product of a single gene that gets translated into a single protein, but some viruses produce mRNAs that contain protein-coding regions from multiple genes separated by an IRES.
This makes the IRES a powerful scientific tool for multigene expression. "As genes linked by an IRES are translated independently, we can adjust their relative expression by varying the strength of the IRES," explains Yang. "However, the number of naturally available IRESs is limited and can only provide a narrow range of gene expression." Yang's team decided to expand their options by generating a library of IRESs with a variety of sequence mutations. Then they assessed how these variants affected relative protein production when inserted between two genes.
To test the different IRES constructs, the researchers produced antibodies—immune proteins formed of large 'heavy-chain' and smaller 'light-chain' protein components. The 24 variants they generated varied considerably in relative gene activity, ranging from a 12 to 96 per cent reduction in the production of light-chain proteins relative to heavy-chain proteins.
These differences had a profound effect on mature antibody assembly. For instance, producing the two components in equal proportions resulted in high levels of antibody production but also yielded many undesirable byproducts. However, an IRES that increased relative light-chain to heavy-chain production by 50 per cent virtually eliminated those byproducts without a meaningful impact on total antibody production.
A United States biotech company has already expressed interest in licensing this strategy for improved antibody production. Meanwhile, Yang's group has additional applications in mind. For example, these sequences could help manipulate glycosylation pathways, a crucial mechanism for functional modification of proteins that depends on finely choreographed activity from multiple enzymes. "This can critically impact the biological activity, serum half-life and immunogenicity of therapeutic proteins," says Yang.
More information: Koh, E. Y. C., Ho, S. C. L., Mariati, Song, Z., Bi, X., Bardor, M. & Yang, Y. "An internal ribosome entry site (IRES) mutant library for tuning expression level of multiple genes in mammalian cells." PLoS ONE 8, e82100 (2014). dx.doi.org/10.1371/journal.pone.0082100
Journal information: PLoS ONE