A new solution for storing hydrogen fuel for alternative energy
Turning the "hydrogen economy" concept into a reality, even on a small scale, has been a bumpy road, but scientists are developing a novel way to store hydrogen to smooth out the long-awaited transition away from fossil fuels. Their report on a new solid, stable material that can pack in a large amount of hydrogen that can be used as a fuel appears in the ACS journal Chemistry of Materials.
Umit B. Demirci and colleagues explain that storing hydrogen in solids is a recent development and a promising step toward building a hydrogen economy. That's the idea originated in the 1970s and promoted by former President George W. Bush that we replace fossil fuels with hydrogen, which can serve as a clean fuel. Although a promising alternative to conventional energy sources, hydrogen has posed a number of technological challenges that scientists are still overcoming. One of those issues has to do with storage.
Previously, researchers were focused on developing hydrogen-containing liquids or compressing it in gas form. Now, solid storage is showing potential for holding hydrogen in a safe, stable and efficient way. In the latest development on this front, Demirci's team looked to a new kind of material.
They figured out a way to make a novel crystal phase of a material containing lithium, boron and the key ingredient, hydrogen. To check how they could get the hydrogen back out of the material, the scientists heated it and found that it released hydrogen easily, quickly and only traces of unwanted by-products.
More information: "Lithium Hydrazinidoborane: A Polymorphic Material with Potential for Chemical Hydrogen Storage" Chem. Mater., Article ASAP. DOI: 10.1021/cm500980b
Abstract
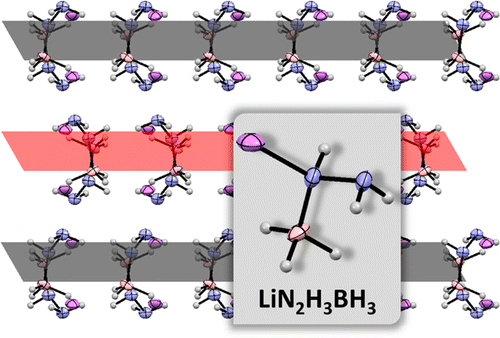
Herein, we describe the synthesis and characterization (chemical, structural, and thermal) of a new crystal phase of lithium hydrazinidoborane (LiN2H4BH3, LiHB), which is a new material for solid-state chemical hydrogen storage. We put in evidence that lithium hydrazinidoborane is a polymorphic material, with a stable low-temperature phase and a metastable high-temperature phase. The former is called β-LiHB and the latter α-LiHB. Results from DSC and XRD showed that the transition phase occurs at around 90 °C. On this basis, the crystal structure of the novel β-LiHB phase was solved. The potential of this material for solid-state chemical hydrogen storage was verified by TGA, DSC, and isothermal dehydrogenations. Upon the formation of the α-LiHB phase, the borane dehydrogenates. At 150 °C, it is able to generate 10 wt % of pure H2 while a solid residue consisting of polymers with linear and cyclic units forms. Reaction mechanisms and formation of bis(lithium hydrazide) of diborane [(LiN2H3)2BH2]+[BH4]− as a reaction intermediate are tentatively proposed to highlight the decomposition of β-LiHB in our conditions.
Journal information: Chemistry of Materials
Provided by American Chemical Society